Products and Phenotyping
Featured Markers and Reagents
When phenotyping regulatory T cells (Tregs) using flow cytometry, several markers are commonly used to identify and characterize these cells. When using surface markers, Tregs are defined by positive expression of CD25 and negative expression of CD127. CD39 and CD73, which are cell surface ecto-nucleotidase enzymes that contribute to the suppressive capacity of Tregs, have also been characterized. Further, activated functional Tregs have also been demonstrated to upregulate LAP (TGF-β1) and GARP, while down-regulating expression of CD45RA.
The combination of the markers below can provide a more comprehensive characterization of Tregs. Researchers frequently use panels with multiple markers to accurately identify and study Tregs in flow cytometry experiments. Furthermore, functional assays and cytokine analysis are necessary to confirm properties of the identified Treg population.
|
Marker1 |
Description |
|
A classical Treg marker, CD25 is the alpha chain of the interleukin-2 receptor. |
|
|
Ectonucleotidases involved in adenosine production and contribute to Treg suppressive function. |
|
|
Integrin expressed by intraepithelial lymphocyte T cells and some peripheral Tregs. |
|
|
Beta chain of the interleukin-7 receptor expressed at low levels by Tregs. |
|
|
Expressed by activated Tregs. It contributes to Treg stability and function. |
|
|
Inhibitory receptor involved in immune response suppression. |
|
|
Checkpoint molecule involved in immune response suppression. |
|
|
Checkpoint molecule involved in immune response suppression. |
|
|
Distinguishes thymic Tregs (tTregs) from peripherally induced Tregs (iTregs). |
|
|
Constitutively expressed on CD4+ CD25+ Tregs, GITR expression increases on all T cell subsets after activation. It is also found on murine neutrophils and NK cells. |
|
|
Transcription factor considered the master regulator of Tregs. |
|
|
Transcription factor expressed in both thymic and peripheral Tregs. |
|
|
Essential for Tregs to differentiate from naïve CD4+ cells and is important in maintaining Treg cell homeostasis. |
Isolate Regulatory T Cells with MojoSort™

Our Mouse CD4+CD25+ Regulatory T Cell Isolation Kits help you isolate Tregs with ease. MojoSort™ is our magnetic separation system for the isolation and purification of cells from heterogeneous populations. Our isolation kits contain a biotin-antibody cocktail and Streptavidin Nanobeads, allowing you to isolate CD4 and CD25 positive T cells with high purity and yield. The magnetically labeled fraction is retained by the use of a magnetic separator. View our isolation protocol for more information.
A single cell suspension from C57BL/6 mouse spleen was prepared for the negative isolation of CD4+ cells and subsequent positive selection of CD25+ cells using the MojoSort™ Mouse CD4+CD25+ Regulatory T Cell Isolation Kit. Left plot: Cells after CD4 negative isolation and CD25 positive selection. Right plot: Cells prior to CD4 depletion. Cells were fixed and permeabilized using the True-Nuclear™ Transcription Buffer Set, then intracellularly stained with anti-mouse FOXP3 Alexa Fluor® 488. Dead cells were excluded by Helix NP™ Blue.
Improve Ex Vivo Recovery of Treg and NKT cells with Treg-Protector™
Our Treg-Protector (anti-ARTC2 Nanobody, ART2.2) is a blocking nanobody against ARTC2, a GPI-anchored, cell surface ADP-ribosyltransferase that is highly expressed by Tregs and natural killer T (NKT) cells. ARTC2 mediates NAD+-induced cell death (NICD) and the proteolytic shedding of CD27 and CD62L. Blocking the activity of ARTC2 with Treg-Protector improves the recovery of Tregs and NKT cells during their isolation.
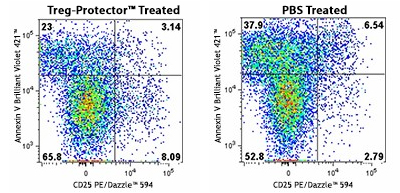
A C57BL/6 mouse was injected (i.v.) with 50 µg of Treg-Protector 15 minutes before harvesting the spleen to isolate lymphocytes (left). The same procedure was done with a control mouse that only received PBS (right). The cells obtained from each mouse were incubated for one hour at 37°C and then were stained with CD3 Brilliant Violet 605™, CD4 FITC, CD25 PE/Dazzle™ 594, and Annexin V Brilliant Violet 421™. Data shown was gated on the CD3+, and CD4+ population. Note the higher frequency of live Tregs (CD4+, CD25+, and Annexin V- T cells) in the mouse that was treated with Treg-Protector (left).
Generate Robust T Cell Activation

Human CD3/CD28 T Cell Activation Beads allow for convenient in vitro activation of human T cells without the need for antigen-presenting cells. Human T cells are stimulated with anti-CD3 and anti-CD28 antibodies coated on superparamagnetic microbeads. Beads are added directly to isolated T cells or to PBMCs for robust T cell activation.
PD-1 expression at day 5 of culture of human CD3+ T cells stimulated with Human CD3/CD28 T Cell Activation Beads. At day of harvest, cells were stained with anti-human CD3 (clone SK7) APC and anti-human CD279 (PD-1) (clone NAT105) Brilliant Violet 421™ and were analyzed by flow cytometry. Cell debris, dead cells, and doublets were excluded from analysis.
Boost Treg Development with Serum Replacements

Treg cells have been implicated in disease progression and in reduction of CAR-T efficacy post-treatment. Thus, it is important to elucidate the role of Treg in cell therapy. Our Cell-Vive™ GMP portfolio provides solutions to investigate the role of Treg cells in a consistent manner. As seen below, when naive T cells are cultured in the presence of CD3/CD28 antibodies and IL-2, our Cell-Vive Chemically Defined Serum Substitute performs on-par with human AB serum, while the Cell-Vive T-NK Xeno-Free Serum Substitute nearly doubles the amount Tregs. Our serum substitutes provide a defined, controlled, and safe alternative for Treg proliferation, mitigating significant risks that are associated with the use of human AB serum.
Human naïve CD4+ T cells were stimulated with Ultra-LEAF™ anti-human CD3 and Ultra-LEAF™ anti-human CD28 antibodies (1 µg/mL), and Recombinant Human IL-2 (200 IU/mL) alone (data not shown) or with the additions of Recombinant Human TGF-β (5 ng/mL) for 6 days in the presence of human AB serum, Cell-Vive™ T Cell CD Serum Substitute, GMP, or Cell-ViveTM T-NK Xeno-Free Serum Substitute. ANOVA was used to determine the effect of serum additives on Treg levels. *p<0.05
FOXP3 and Flow Cytometry Treg Kits
FOXP3 is a transcription factor which is proposed to be a master regulatory gene and a more specific marker of T regulatory cells compared to common T cell surface markers (such as CD4, CD25, and CD127). Transduction of FOXP3 in CD4+/CD25- mouse cells has been shown to induce GITR, CD103, and CTLA-4 and induce a T regulatory cell phenotype2. Overexpression of FOXP3 in mice has been shown to lead to a hypoactive immune state suggesting that this transcriptional factor is a central regulator of T cell activity3. In humans, unlike in mice, two isoforms of FOXP3 have been reported: one corresponding to the canonical full-length sequence; the other (FOXP3 delta 2) lacking exon 2(4).
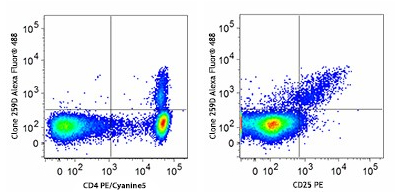
Our flow cytometry kits and FOXP3 buffers provide valuable tools for studying T cell populations and immune responses. Designed for both human and mouse cells, our True Nuclear™ Treg flow cytometry kits contain optimized antibody and reagent concentrations providing excellent performance and convenience in multicolor fluorescence experiments.
Human peripheral blood lymphocytes were stained with True-Nuclear Human Treg Flow™ Kit (FOXP3 Alexa Fluor® 488/CD4 PE/Cyanine5/CD25 PE).
Helios

Helios is a member of the Ikaros family of transcription factors. It binds to the FOXP3 promoter, stabilizing FOXP3 expression and enhancing Treg suppressive function. In mice and humans, Helios is expressed in approximately 70-80% of Treg cells, particularly in those arising from the thymus (tTregs) rather than peripherally induced Tregs (iTregs)5,6. Although initially considered a marker to distinguish naturally occurring Tregs, Helios expression is also associated with T cell activation and proliferation, regardless of the cell subset involved.
C57BL/6 mouse splenocytes were surface stained with anti-mouse CD4 APC and then fixed and perm using True-Nuclear Transcription Factor Buffer Set. Cells were then intracellularly stained with purified anti-Helios recombinant (clone QA20A52) (left) or rat IgG1, κ isotype control (right) followed by anti-rat IgG1 PE.
References
1. La Cava, Antonio. “Natural Tregs and autoimmunity.” Frontiers in bioscience (Landmark edition) vol. 14,1 333-43. 1 Jan. 2009, doi:10.2741/3247
2. Wu, Yongqing et al. “FOXP3 controls regulatory T cell function through cooperation with NFAT.” Cell vol. 126,2 (2006): 375-87. doi:10.1016/j.cell.2006.05.042
3. Hori, Shohei, and Shimon Sakaguchi. “Foxp3: a critical regulator of the development and function of regulatory T cells.” Microbes and infection vol. 6,8 (2004): 745-51. doi:10.1016/j.micinf.2004.02.020
4. Allan, Sarah E et al. “The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs.” The Journal of clinical investigation vol. 115,11 (2005): 3276-84. doi:10.1172/JCI24685
5. Akimova, Tatiana et al. “Helios expression is a marker of T cell activation and proliferation.” PloS one vol. 6,8 (2011): e24226. doi:10.1371/journal.pone.0024226
6. Thornton, Angela M et al. “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells.” Journal of immunology vol. 184,7 (2010): 3433-41. doi:10.4049/jimmunol.0904028
 Login/Register
Login/Register 






Follow Us