- Other Names
- Separation Buffer, Isolation Buffer
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

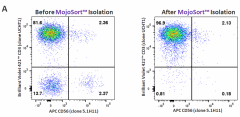
Human CD3+ cells were isolated from Peripheral Blood Mononuclear cells using Cell-Vive™ CD Cell Separation Buffer (Cat. 420512). The cells were isolated using a MojoSort™ magnet (Cat. 480019) as indicated in the protocol for MojoSort™ Human CD3 T cell Isolation Kit (Cat. 480131) (A) or using a common commercially available magnetic separation column kit from another supplier (B). Purity was verified, before and after isolation, by staining with Brilliant Violet 421™ anti-human CD3 (Clone, UCHT1, Cat. 300434) and APC anti-human CD56 (Clone 5.1H11, Cat. 362504). -

| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 420512 | 250 mL | 250 CHF | ||||
Cell-Vive™ CD Cell Separation Buffer, GMP is a cell separation buffer with a chemically-defined formulation without animal-derived components or preservatives. This product is intended to be used with our MojoSort™ magnetic cell separation system or equivalent cell separation products. Cell-Vive™ CD Cell Separation Buffer, GMP is filtered through a 0.2 μm membrane and is a 1X formulation ready to use. This GMP product is for research and further cell-based bioprocessing use only
Product DetailsBioLegend Cell-Vive™ GMP cell culture products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated GMP facility compliant with ISO 13485:2016. Specifications and processes include
- Low endotoxin level (< 1 EU/mL)
- Mycoplasma and bacterial/fungal growth testing
- Batch-to-batch consistency
- Vendor qualification
- Raw material traceability and documentation
- Documented procedures and employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- QA review of released products
- Quality audits per ISO 13485:2016
Product Details
- Formulation
- Serum-Free, Chemically-Defined, no Sodium Azide
- Endotoxin Level
- < 1 EU/mL
- Storage & Handling
- Store between 2°C - 8°C
- Application
-
Cell Separation - Verified
- Application Notes
-
Use of Cell-Vive™ CD Cell Separation Buffer, GMP is recommended for cell separation using MojoSort™ nanobeads or equivalent separation kits. Cell-Vive™ CD Cell Separation Buffer, GMP is a serum-free chemically-defined formulation without animal derived components such as BSA. Cell-Vive™ CD Cell Separation Buffer, GMP is filtered through a 0.2 μm membrane and is a 1X formulation ready to use. Use the buffer under aseptic conditions as needed.
- Additional Product Notes
-
Chemically-Define formulation Cell-Vive™ CD Cell Separation Buffer, GMP can be used for cell separation following MojoSort™ isolation protocol or equivalent isolation kit protocols.
- Disclaimer
-
BioLegend Cell-Vive™ GMP Cell Culture products are for research use only. Suitable for ex vivo cell processing. Not for injection or diagnostic or therapeutic use. Not for resale. BioLegend will not be held responsible for patent infringement or other violations that may occur with the use of our products
Antigen Details
- Gene ID
- NA
 Login / Register
Login / Register 














Follow Us