Advancing Neurodegenerative Disease Research With Novel Reagents
Neurodegenerative disorders result from the loss or dysfunction of neurons in the nervous system, which can have debilitating effects like dementia, memory loss, and ataxia. Therapies and treatments for these diseases are limited, raising the need to advance research into the causes of these disorders.
Gain a better understanding of the mechanisms and origins of neurodegenerative disorders with our resources for neuroscience research. Our antibodies, recombinant proteins, immunoassays, and reagents are optimized to study protein aggregation and cellular processes involved in neurodegeneration.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with limited treatment options for symptoms but currently no cure. PD is a characterized by progressive problems with movement and balance. It is the second most common neurodegenerative disorder after Alzheimer’s disease (AD)1.
April is PD awareness month, and we’re highlighting the resources our scientists have developed to advance research, including high-quality and specific reagents for the detection of key target proteins involved in the pathogenesis of PD and AD.
To learn more about the known associated proteins that contribute to PD, and a comprehensive list of available reagents, visit our Parkinson’s disease page.
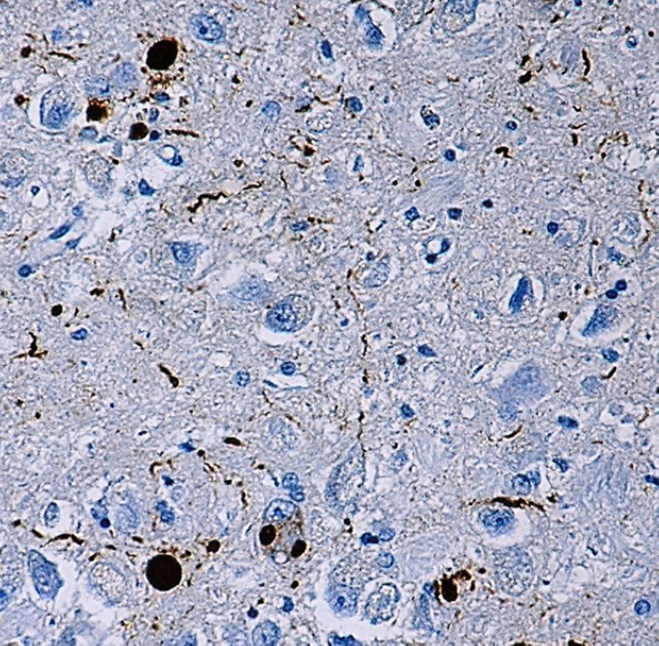
IHC staining of α-synuclein (clone Syn514) on formalin-fixed paraffin-embedded Parkinson's disease tissue. After antigen retrieval, the tissue was incubated with the primary antibody, followed by Ultra Streptavidin HRP Detection Kit (Multi-Species, DAB), and hematoxylin and bluing solution counterstain.
α-Synuclein is a 140 amino acid neuronal protein that belongs to the Synuclein family, which also includes β-Synuclein and γ-Synuclein. At the cellular level, α-Synuclein primarily localizes in the synaptic terminals of neurons. Although the normal function of α-Synuclein is not fully understood2, it was reported to have an important role in synaptic vesicle transport and membrane fusion3. Moreover, the aggregate form of α-Synuclein is one of the major components of inclusion bodies (Lewy bodies) inside nerve cells affected by Parkinson’s disease and Lewy body dementia.
The aggregated and fibrillar forms of α-Synuclein are known to be highly toxic, interfering with cellular functions that lead to neurodegeneration. Furthermore, the ring-like pores can damage membrane integrity and disturb intracellular calcium homeostasis, contributing to neuronal toxicity4. α-Synuclein peptides can also be found as components of amyloid plaques in AD, as well as in glial cytoplasmic inclusions in Multiple System Atrophy5.
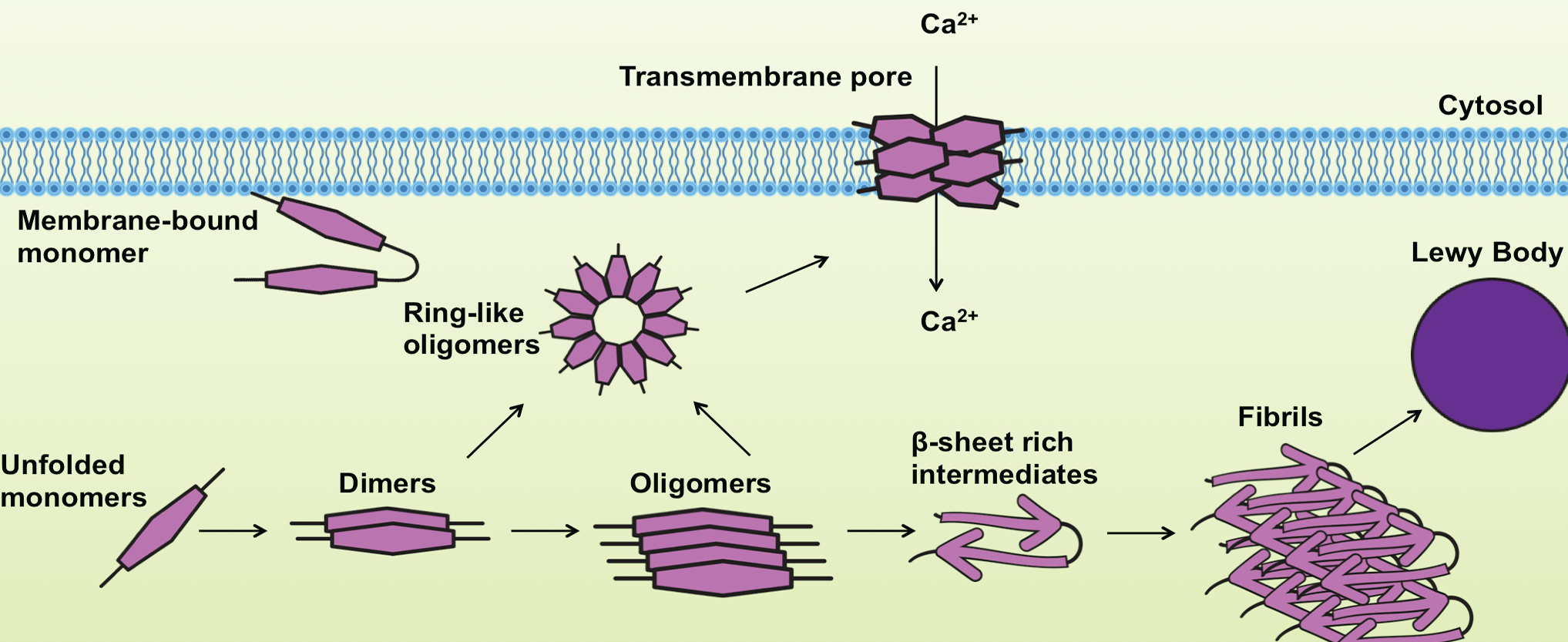
Adapted from Lashuel et al., Nature Neuroscience Reviews 2013.
Levels of α-Synuclein in cerebrospinal fluid (CSF) or plasma are currently being investigated as a potentially useful biomarker for disease diagnosis or prognosis6. Identifying and precisely measuring biomarkers associated with diseases is crucial for neurodegeneration research.
 Accurately quantify α-Synuclein aggregates in tissue and cell lysates with our newly launched LEGEND MAX™ α-Synuclein Aggregate ELISA Kit. This research tool was designed in collaboration with The Michael J. Fox Foundation, and includes pre-coated plates and ready-to-use reagents for the detection of aggregated α-Synuclein.
Accurately quantify α-Synuclein aggregates in tissue and cell lysates with our newly launched LEGEND MAX™ α-Synuclein Aggregate ELISA Kit. This research tool was designed in collaboration with The Michael J. Fox Foundation, and includes pre-coated plates and ready-to-use reagents for the detection of aggregated α-Synuclein.
Our LEGEND MAX™ Human α-Synuclein Aggregate and α-Synuclein (Colorimetric) ELISA Kits are specifically designed for the accurate quantitation of both aggregated and total human α-Synuclein from various sample types, including CSF, serum, plasma, and cell culture supernatant. These new kits offer ready-to-use reagents with a higher sensitivity to low levels of α-Synuclein (as low as 1.8 pg/mL).
We also provide resources and reagents for advancing research in other neurodegenerative disorders such as AD. The accumulation of amyloid β peptide (Aβ (1-42)) in extracellular plaques is one of the hallmarks of AD7. Aβ (1-40) is also an important biomarker for AD. Although Aβ (1-40) is more abundant than Aβ (1-42), it has a lower tendency to aggregate. The Aβ42/Aβ40 ratio in CSF is an important diagnostic biomarker of the severity of disease8. A decrease in Aβ40 CSF levels has also been implicated in other conditions like frontotemporal dementia. Our LEGEND MAX™ Human Amyloid β 1-42 and LEGEND MAX™ Amyloid β 1-40 ELISA kits are designed for human amyloid protein fragment quantification in biological samples. Both LEGEND MAX™ ELISAs are analytically validated and can be used to detect native proteins in several types of human samples, including serum, plasma, and CSF.
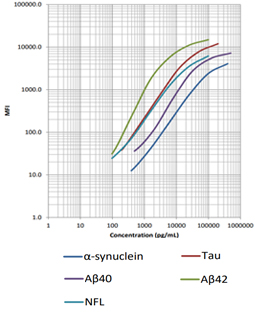 Our LEGENDplex™ Human Neurodegenerative Disease Biomarker Panel is designed to measure five neurodegenerative biomarkers simultaneously (α-Synuclein, Tau, Aβ40, Aβ42, and Neurofilament-L). This panel is a multiplex bead-based immunoassay that utilizes fluorescence-encoded beads suitable for use on various flow cytometers. It provides higher detection sensitivity with broader dynamic ranges than the traditional ELISA method.
Our LEGENDplex™ Human Neurodegenerative Disease Biomarker Panel is designed to measure five neurodegenerative biomarkers simultaneously (α-Synuclein, Tau, Aβ40, Aβ42, and Neurofilament-L). This panel is a multiplex bead-based immunoassay that utilizes fluorescence-encoded beads suitable for use on various flow cytometers. It provides higher detection sensitivity with broader dynamic ranges than the traditional ELISA method.
In addition to these immunoassays, we have expanded our portfolio to include aggregate preferring antibodies and phospho-specific antibodies for α-Synuclein. α-Synuclein is phosphorylated at low levels under normal physiological conditions whereas the majority of the protein is phosphorylated in Lewy bodies at S129.
Our α-Synuclein antibody sampler kits offer flexibility for sampling and detection of key targets in neurodegeneration. Our sampler kits are ideal for visualization of native and post-translationally modified forms of these proteins, and provide specificity for detection of full-length, truncated and phosphorylated species of α-Synuclein.
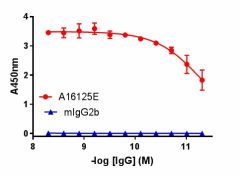
Direct ELISA of IHC staining of anti-DJ-1 (PARK7) antibody (clone A16125E) and isotype-matched control anti-mouse IgG2b antibody binding to plate-immobilized recombinant human DJ-1. Followed by incubation with HRP goat anti-mouse IgG antibody. TMB Substrate Set was used for detection.
In addition to α-Synuclein pathology, researchers have identified more than 25 PARK7 gene mutations that can cause PD. These mutations are associated with the early-onset form of the disorder, which begins before age 50. PARK7 acts as a protease, an oxidative stress sensor, and as a redox-sensitive chaperone in protein folding9.
We are committed to empowering Parkinson’s disease research by providing you resources for key targets and continuing to develop research tools for all aspects of your neurodegeneration research. Make a difference in your neuroscience research by trusting the high-quality and validated reagents created by our scientists.
References:
- Lebouvier, Thibaud et al. “The second brain and Parkinson's disease.” The European journal of neuroscience vol. 30,5 (2009): 735-41. DOI:10.1111/j.1460-9568.2009.06873.x
- Fakhree, Mohammad et al. “Cooperation of Helix Insertion and Lateral Pressure to Remodel Membranes.” Biomacromolecules vol. 20,3 (2019): 1217-1223. DOI:10.1021/acs.biomac.8b01606
- Burré, Jacqueline. “The Synaptic Function of α-Synuclein.” Journal of Parkinson's disease vol. 5,4 (2015): 699-713. DOI:10.3233/JPD-150642
- Lashuel, Hilal A et al. “The many faces of α-synuclein: from structure and toxicity to therapeutic target.” Nature reviews. Neuroscience vol. 14,1 (2013): 38-48. DOI:10.1038/nrn3406
- Koh, Yong Hui et al. “Patient-Derived Induced Pluripotent Stem Cells and Organoids for Modeling Alpha Synuclein Propagation in Parkinson's Disease.” Frontiers in cellular neuroscience vol. 12 413. (2018). DOI:10.3389/fncel.2018.00413
- Culvenor, J G et al. “Non-Aβ component of Alzheimer's disease amyloid (NAC) revisited. NAC and alpha-synuclein are not associated with Aβ amyloid.” The American journal of pathology vol. 155,4 (1999): 1173-81. DOI:10.1016/s0002-9440(10)65220-0
- Jin, Sha et al. 2016. Amyloid-β(1-42) Aggregation Initiates Its Cellular Uptake and Cytotoxicity. The Journal of biological chemistry vol. 291,37: 19590-606. DOI:10.1074/jbc.M115.691840
- Hansson, O., Lehmann, S., Otto, M. et al. 2019. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alz Res Therapy 11, 34. DOI:10.1186/s13195-019-0485-0
- Abou-Sleiman, Patrick M et al. “The role of pathogenic DJ-1 mutations in Parkinson's disease.” Annals of neurology vol. 54,3 (2003): 283-6. DOI:10.1002/ana.10675
 Login/Register
Login/Register 






Follow Us