Introduction
This protocol is applicable to the use of both MojoSort™ Mouse Dead Cell Removal and MojoSort™ Human Dead Cell Removal kits on commercially available magnetic columns available from other vendors. BioLegend MojoSort™ nanobeads work in commonly used separation columns, based on our internal research as well as validation by external testing by academic labs. This simple protocol consists of following the MojoSort™ protocol to label the cells with pre-diluted MojoSort™ reagents and using the columns as indicated by the manufacturer.
Note: Due to the properties of our beads, it may be possible to use far fewer beads than with other commercial suppliers. We recommend a titration to find the best dilution factor. However, as a general rule, dilutions ranging from 1:3 to 1:20 for the Nanobeads can be used.
Reagent List
|
Important Note
MojoSort™ magnetic particles can be used with other commercially available magnetic separators, both free standing magnets and column-based systems. Because MojoSort™ protocols are optimized for the MojoSort™ separator, the protocols may need to be adjusted for other systems. Please contact BioLegend Technical Service (tech@biolegend.com) for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Protocol Steps
- Prepare cells from your tissue of interest or blood without lysing erythrocytes.
- In the final wash of your sample preparation, resuspend the cells in MojoSort™ Buffer by adding up to 4 mL in a 5 mL (12 x 75 mm) polypropylene tube.
Note: Keep MojoSort™ Buffer on ice throughout the procedure.
- Filter the cells with a 70 µm cell strainer, centrifuge at 300xg for 5 minutes, and resuspend in an appropriate volume of MojoSort™ Buffer. Count and adjust the cell concentration to 1 x 108 cells/mL
- If working with fewer than 1x107 cells, resuspend in 100µL sample volume.
- If working with fewer than 1x107 cells, resuspend in 100µL sample volume.
- Preassemble the monomer-streptavidin complex:
- For each sample, add 10µL of pre-diluted Apo-Monomer and 10µL of pre-diluted Streptavidin Nanobeads in the same tube. Mix well by pipetting up and down several times. Scale up volumes for each component respectively if separating multiple samples
- Incubate at room temperature for 5 minutes then proceed to next step.
- Aliquot 100µL of cell suspension (1x107 cells, or fewer) into separate tubes for each sample. Add 20µL of the monomer-streptavidin complex (from step 4) into each tube. Mix well and incubate on ice for 15 minutes.
- Scale reagent use to cell count accordingly. We observed higher purity and yield from samples containing fewer cells (~1x106 cells, from cryopreserved PBMCs) with adding 8x less monomer-streptavidin complex (2.5µL). Some optimization may be required.
- Scale reagent use to cell count accordingly. We observed higher purity and yield from samples containing fewer cells (~1x106 cells, from cryopreserved PBMCs) with adding 8x less monomer-streptavidin complex (2.5µL). Some optimization may be required.
- Wash the cells by adding MojoSort™ Buffer up to 4mL. Centrifuge the cells at 300xg for 5 minutes.
- Add the appropriate amount of MojoSort™ Buffer and proceed to separation. At least 500 µL is needed for column separation.
Columns:
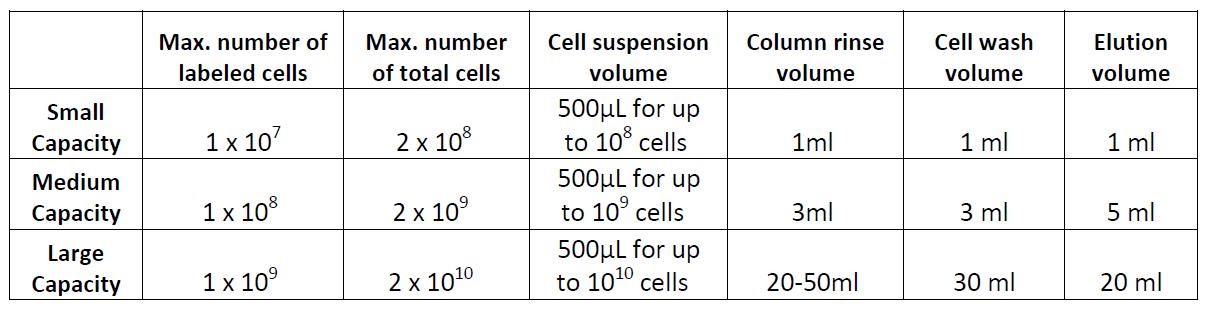
Example of magnetic separation with medium capacity columns:
- Place the column in a magnetic separator that fits the column.
- Rinse the column with 3 mL of cell separation buffer.
- Add the labeled cell suspension to the column through a 30 µm filter and collect the fraction containing the unlabeled cells. These are the live cells.
- Wash the cells in the column 3 times with 3 mL of buffer and collect the fraction containing the unlabeled cells. Combine with the collected fraction from step 3. These are also live cells.
- (optional) Take away the column from the magnet and place it on a tube. Then add 5 mL of buffer and flush out the magnetically labeled fraction with a plunger or supplied device. This fraction contains the dead cells – these cells may be useful as controls to monitor purity/yield, or other purposes.
 Login/Register
Login/Register 






Follow Us