- Regulatory Status
- RUO
- Other Names
- Control Cells
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

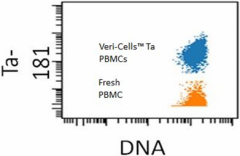
Unlabeled cells may be differentiated using the tantalum signal. Data provided by Adeeb Rahman, PhD. Mt. Sinai Human Immune Monitoring Center, Hess Center for Science and Medicine. -

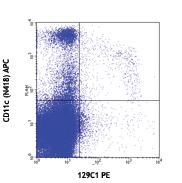
Scatter profile (left) and staining for CD3 (clone UCHT1) FITC and CD19 (clone HIB19) APC (right) on Veri-Cells™ Heavy Metal (Ta) PBMC. CD3 and CD19 staining shown on lymphocyte population (gate on left scatter plot).
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 427201 | 10 tests | 165€ | ||||
| 427203 | 50 tests | 672€ | ||||
The reconstituted Veri-Cells™ Heavy Metal Ta PBMC are intended to offer mass cytometry laboratories stabilized human blood cells which have been labeled with the heavy metal tantalum. When mixed with sample cells suspensions, the cells of interest can be differentiated from the labeled control PBMCs using the tantalum signal with minimal variability across experiments. These cells have been verified to work with commonly tested cell surface markers such as CD3, CD4, CD8, CD14, CD16, CD19, CD38, CD56, CD123 and HLA-DR.
This product contains human cells, a potentially biohazardous material. Blood used in preparation of these samples was tested and found to be negative for antibody and nucleic acid testing against human immunodeficiency virus (HIV), Hepatitis B and C virus, HTLV (Human T-lymphotrophic virus I and II), surface antigen for Hepatitis B (HBsAg) virus, syphilis and West Nile Virus using FDA approved methods. Biological tests are not 100% accurate. Use standard precautions when handling, and treat as if the product is capable of transmitting disease. When handling or disposing, follow precautions described in CDC and FDA recommendations and OSHA Bloodborne Pathogen guidelines.
Kit Contents
- Kit Contents
-
Cat. No. 427201
- 2 vials of Veri-Cell™ Heavy Metal (Ta) PBMC (approximately 1 X 106 cells per vial)
- 1 vial of 0.7 ml Veri-Cells™ Buffer A Plus
Cat. No. 427203
- 10 vials of Veri-Cell™ Heavy Metal (Ta) PBMC (approximately 1 X 106 cells per vial)
- 5 vials of 0.7 ml Veri-Cells™ Buffer A Plus
Veri-Cells™ Buffer A Plus is an improved version of the original Veri-Cells™ Buffer A to improve staining performance with tandem dyes.
Product Details
- Formulation
-
Cat. No. 427201: 2 vials each containing ≥ 1 X 106 lyophilized human peripheral blood mononuclear cells (PBMC).
Cat. No. 427203: 10 vials each containing ≥ 1 X 106 lyophilized human peripheral blood mononuclear cells (PBMC). - Preparation
- The Veri-Cells™ Heavy Metal Ta PBMC kit is prepared from 2 vials of lyophilized human PBMCs labeled with tantalum and a vial of Veri-Cells™ Buffer A Plus.
- Storage & Handling
- Store the kit at 2°C - 8°C upon receipt. Do not open sealed pouch until ready to use. Once reconstituted, the cells may be used for up to five days if properly stored at 2°C - 8°C in the buffer provided.
- Application
-
FC, CyTOF® - Quality tested
- Recommended Usage
-
50 µL per test (after reconstitution with 325 µL of Veri-Cells™ Buffer A Plus)
Once reconstituted, let the cells sit for 15 minutes at room temperature prior to staining.
Veri-Cells™ Buffer A Plus may show precipitation over time, however this is normal and does not affect performance of the buffer. - Application Notes
-
The Veri-Cells™ Heavy Metal Ta PBMC kit contains stabilized human blood cells which have been pre-labeled with the metal tantalum (Ta). The reconstituted human PBMCs can be added to test samples as a control for CyTOF applications. The cells have been verified to work with commonly tested cell markers such as CD3, CD4, CD8, CD14, CD16, CD19, CD38, CD56, CD123 and HLA-DR. Assessing the suitability of this product as a control for the end user’s molecule of interest is recommended. The tantalum signal is confirmed by a CyTOF Helios or equivalent mass cytometry instrument.
Cell subset
Acceptance Criteria, % positive of lymphocyte gate
CD3+
55-95
CD4+CD3+
30-65
CD8+CD3+
10-40
CD19+CD3 –
3-30
CD56+CD16+CD3 –
3-40
Note on indicated ranges: Percent positive values are obtained based on gates set on unstained cells. The antibodies used for testing were the following: CD56 PE (clone 5.1H11), CD16 PE (clone 3G8), CD19 APC (clone HIB19), CD3 FITC (clone UCHT1), CD4 PE/Cy7 (clone RPA-T4), and CD8 APC/Cy7 (clone RPA-T8). Variability in antibodies used, instruments, staining conditions and other factors may influence end-user values. The Certificate of Analysis specific values are provided as a reference. It is recommended that individual laboratories establish its own control values and ranges for each parameter based on laboratory specific conditions and protocols.
Please use our CoA Look-Up Tool to find the expected lot-specific frequencies of certain cell populations included within each lot of Veri-Cells™ Heavy Metal (Ta) PBMC. - Additional Product Notes
-
Veri-Cells™ Buffer A Plus is an improved version of the original Veri-Cells™ Buffer A to improve staining performance with tandem dyes.
Antigen Details
- Gene ID
- NA
Related Pages & Pathways
Pages
Related FAQs
- Is washing required after staining?
-
Yes, it is recommended to wash after staining. We recommend using BioLegend's Cell Staining buffer (Cat. No. 420201) for the wash step.
- Can the cells be activated after rehydration?
- No, they cannot be activated.
- Are these cells virus/pathogen free?
- These are tested to be free of HIV, HBV, syphilis, and HCV.
- Are there any RBCs in Veri-cells™?
- There may be a few, but the majority of RBCs are not present in Veri-Cells™ preparations.
- Are these cells viable?
- No they are not.
- Can a constant percentage of each population be expected in each lot?
- Yes, within each lot, cell populations remain constant.
- Are the cells fixed?
-
They are treated with our proprietary mixture prior to lyophilization.
- Can they be used to detect intracellular cytokines such as TNF-α or IFN-γ?
-
For this, use Veri-Cells™ Activated (Cytokine) PBMC which have been activated with Cell Activation Cocktail (with Brefeldin A) containing PMA and Ionomycin. These have been validated to stain positive for activation-induced cytokines including TNF-α and IFN-γ. Veri-Cells™ PBMC and Veri-Cells™ CD4-Low PBMC will not have detectable TNF-α or IFN-γ.
- How do we QC these cells?
-
Each Veri-Cells™ product is tested with a panel of antibodies to ensure expression of these markers falls within the expected range. For more information regarding the exact markers used for each product, please contact technical service.
 Login / Register
Login / Register 













Follow Us