Flex-T™ Tetramer Preparation and Flow Cytometry Staining Protocol
Background
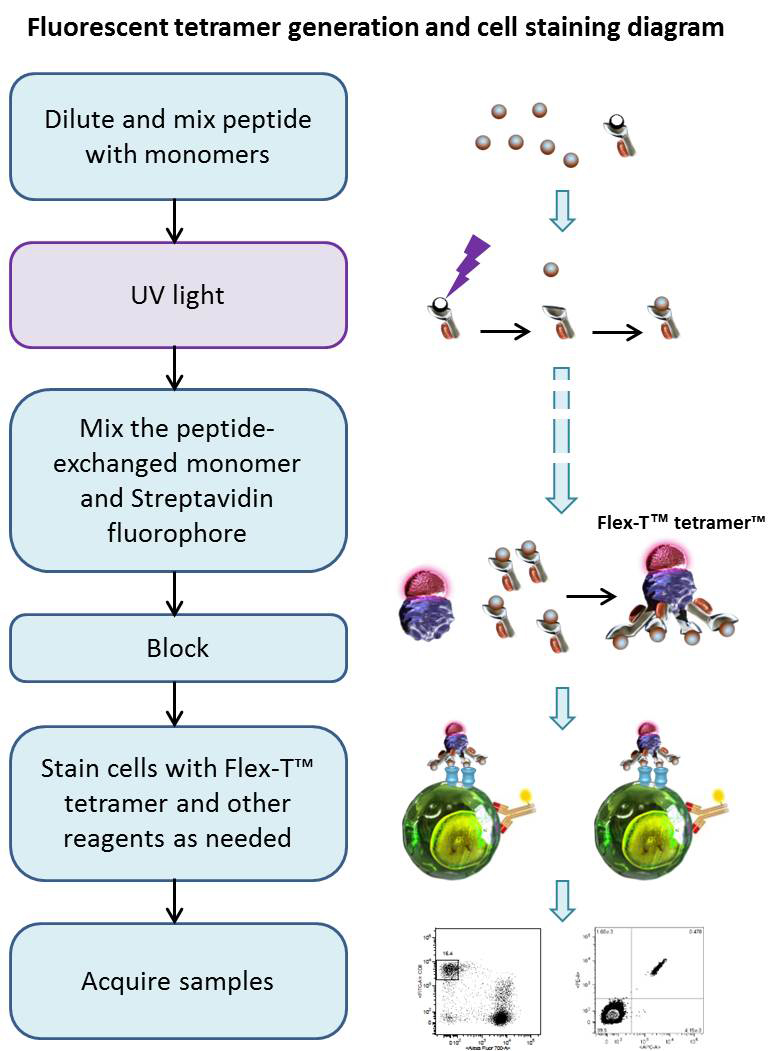
Using UV-induced peptide exchange, MHC/peptide monomers can be generated with conditional Flex-T™ monomers that harbor peptides of interest in their binding grooves. These new MHC monomers are subsequently multimerized using streptavidin-fluorophore conjugates. The resulting Flex-T™ reagents can be used for staining antigen-specific T cells and flow cytometric analysis. In humans, the MHC molecules are called HLA (Human Leukocyte Antigen).
Reagents
- Phosphate buffered saline pH 7.4, 10X concentrate (PBS, BioLegend Cat. 926201)
- Peptide Flex-T™ monomer UVX
- DMSO (e.g. Sigma-Aldrich Cat#D5879)
- 50mM D-Biotin (e.g. Thermo Fisher, Cat#B20656)
- 10% (w/v) NaN3 (e.g Sigma, Cat#S2002)
- Fluorophore-conjugated Streptavidin (BioLegend Cat. 405203, Cat. 405207, Cat. 405225 or equivalent)
- Cell Staining Buffer (BioLegend Cat. 420201 or equivalent)
- 96-well Polypropylene Microplate, V-shape (e.g. Greiner bio-one cat. # 651201)
- 96-well Polystyrene Microplate, U-shape (e.g. Falcon Cat#353077) or 5mL, 12 x 75mm tubes (e.g. Falcon Cat# 352008)
- Plate sealers (BioLegend Cat. 423601)
- 1.5ml tubes (e.g. Eppendorf Cat# 022364111)
- For proteogenomic applications, compatible with our TotalSeq™ product line, use one of our oligo barcoded fluorophore-conjugated Streptavidin reagents
Equipment
- UV Lamp, long-wave UV source (365nm, 8 Watts, or similar). The light source should allow sample to be placed 2 – 5 cm away from the lamp.
- Incubator (37°C)
- Centrifuge capable of accommodating microtiter plates and tubes
- Single and multichannel pipettes capable of accurate delivery of variable volumes, and pipette tips
Tips
- DMSO can be used to dissolve the peptides. However, do not exceed an end concentration of 10% (v/v) in the exchange reaction.
- Avoid repeated freeze-thawing.
- The Flex-T™/peptide solution needs to be kept on ice in the dark as much as possible. Do not work in front of a window.
- The use of short-wavelength (254 nm) or broad-band UV lamps is detrimental to MHC complexes.
- Centrifuge all vials before use (1 minute 2500 x g at 4°C).
Procedure
Peptide Exchange:
- Bring all reagents to 0°C by putting them on ice.
- Dilute 10mM stock solutions of peptides of choice to 400µM by mixing 5µl of peptide stock solution with 120µl PBS, and keep on ice.
- Add 20µl diluted peptide (400µM) and 20µl peptide Flex-T™ monomer UVX (200µg/ml) into 96-well V bottom plate. Mix by pipetting up and down.
- Seal the plate; centrifuge at 2500xg for 2 minutes at 4°C to collect the liquid down
- Remove the seal; put the plate on ice and illuminate with UV light for 30 minutes (the distance of the UV lamp to the samples should be 2-5cm).
- Seal the plate; incubate for 30 minutes at 37°C in the dark.
- To evaluate the efficiency of the peptide exchange follow the Protocol for HLA class I ELISA to evaluate peptide exchange.
Generation of Tetramers:
- Transfer 30µl of peptide-exchanged monomer into a 1.5ml Eppendorf tube, or a new plate, then add 3.3µl of conjugated streptavidin, mix by pipetting up-and-down. Incubate on ice in the dark for 30 minutes. This is enough for about 15 tests.
Note: BioLegend fluorophore-conjugated streptavidin products are recommended. For 30µl of exchanged Flex-T™ monomer we suggest using 3.3µl of BioLegend PE-streptavidin (Cat#405203) or APC- streptavidin (Cat#405207). For BV421-streptavidin conjugate (Cat#405225) use 1.3µl. For oligo barcoded Streptavidin reagents please use 1.3µl. For optimal reaction with other fluorophore-conjugated streptavidin products ensure that the monomer:streptavidin conjugate has a 5:1 ~ 6:1 molar ratio.
(Note that purified, biotinylated, HRP, MojoSort™, and Ultra Streptavidin (USA) kits are not recommended for this procedure.) - During the incubation, prepare blocking solution by adding 1.6µl 50mM D-Biotin and 6µl 10% (w/v) NaN3 to 192.4µl PBS, mix by vortexing. After the incubation, add 2.4µl of blocking solution and pipette up-and-down to stop the reaction.
- Incubate the tubes or sealed plates at 2-8°C overnight (or on ice for 30 minutes in the dark, if staining needs to be performed immediately).
Tip: We recommend Flex-T™ to be assembled with two different streptavidin conjugates in separate reactions. This allows for two-color staining with the same tetramer allele, ensuring the highest specificity.
Cell Staining and Flow Cytometric Analysis:
- Prepare cells of interest
- Prior to performing staining, centrifuge the assembled tetramers in tube or plate at 2500xg for 5 minutes at 4°C. Then keep on ice in the dark.
- Add 2 x 106 cells to 12 x 75mm tubes or a 96-well U-bottom plate. Adjust volume to 200µl with Cell Staining Buffer. Add 2µl per sample of Flex-T™ complex prepared in Steps 7-9, mix and incubate on ice in the dark for 30 minutes.
- If co-staining with surface antibodies, prepare the antibody cocktail based on optimal staining concentration of each reagent. Incubate for 30 minutes on ice in the dark.
- Wash the cells with Staining Buffer two times. Resuspend cells with Staining Buffer.
- Acquire the samples with a flow cytometer and appropriate settings within 2 hours.
Tip: A titration of the Flex-T™ is recommended for optimal performance.
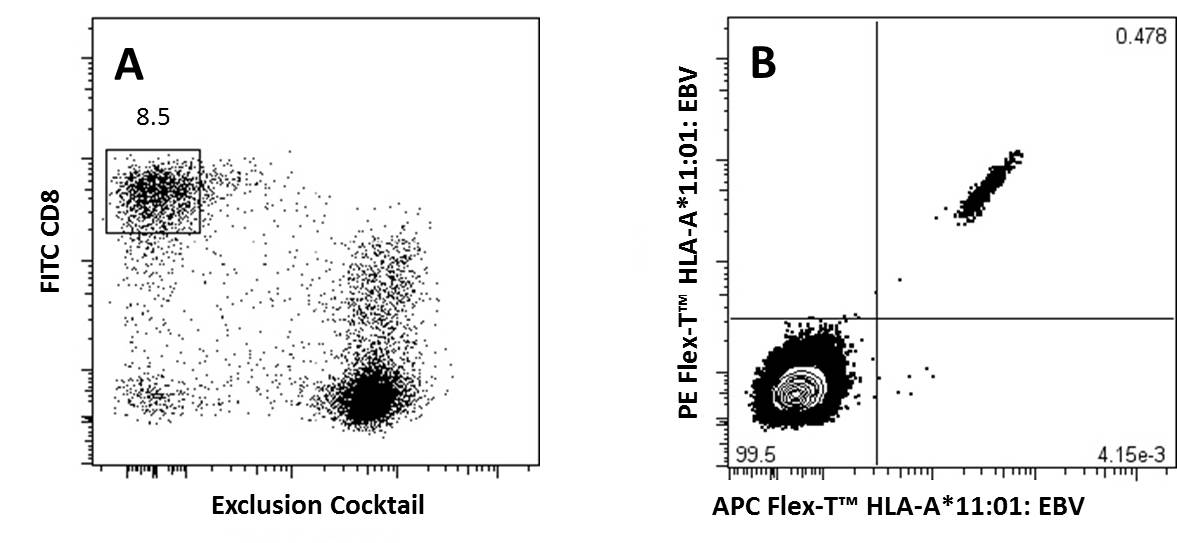
Representative Data:
A) CD8+ T Cells previously gated on lymphocytes (FSC vs SSC) and 7-AAD negative events, were stained with FITC anti-CD8a and an exclusion cocktail containing Alexa Fluor® 700 anti-CD4, CD19, CD14, and CD16.
B) Antigen specific CD8+ T Cells, gated as described, were detected with Flex-T™ tagged with PE and APC. HLA-A*11:01 Flex-T™ was loaded with an EBV peptide (IVTDFSVIK).
Chart Protocol
 Login/Register
Login/Register 







Follow Us