Whole Mouse Brain Processing for Microglia Isolation, Cell Separation, and Flow Cytometry
Reagent and Instrument List
|
|
Protocol
Note: Use aseptic techniques if you need to sort and grow cells in culture.
Harvesting the brain
- If the mouse is >2 weeks old, perfuse the mouse with 10 mL 1X PBS through the left ventricle prior to brain harvest.
- Beginning from the brain stem, cut upward along the sagittal suture as to not damage the brain.
Note: Sagittal Suture is a connective joint that splits the two halves of the skull. - Peel the two halves of the skull away to the side.
- Using tweezers, scoop out the brain and place into a petri dish with PBS.
Enzymatic digestion
Note: Due to the effect of trypsin digestion on certain surface antigen epitopes, you may use a dounce homogenizer in lieu of trypsinization in step 7. See Garcia JA et al. below for additional information on this procedure.
- Gently separate the brain into 6-8 pieces.
- Transfer the segments of the brain into a 50 mL polypropylene conical tube.
- Add 10 mL of trypsin to the tube containing the brain, and incubate at 37°C in an incubator or water bath. Every 5 minutes gently shake and invert the tube or gentle pipette the tissue by using 10 mL or 50 mL pipette.
Note: Do NOT leave in trypsin for more than 15 minutes. - After 10 – 15 minutes, triturate the brain clumps until mostly dissociated and add 20-30 mL of complete RPMI. To triturate, you may pipet up and down using either 12 or 50 mL serological pipettes, or glass pipettes.
- Filter the suspension through a 70 μm filter several times (3-4X) and manually gently homogenize the remainder of the clumps.
- Spin at 365 x g for 5 minutes at room temperature, filter through a 70 μm filter and resuspend with appropriate buffer.
- If you wish to remove myelin, resuspend the cell pellet in 4 mL per brain of 37% SIP at room temperature (see the next section for steps on myelin removal). Otherwise, proceed to downstream cell separation, fluorescence activated cell sorting (FACS), flow cytometry analysis (view our recommended surface and intracellular flow cytometry staining protocols), or other applications of choice. If you do not wish to remove myelin, resuspend in PBS and leave on ice.
Optional steps: Myelin Removal for Microglia, Leukocyte Isolation
Prepare several percoll gradient solutions (warmed to room temperature):
- Stock Isotonic Percoll (SIP): Mix 1 part 10X PBS and 9 parts percoll.
- 70% percoll: 7 mL SIP + 2.0 mL 1X PBS
- 37% percoll: 3.7 mL SIP + 5.3 mL 1X PBS
- 30% percoll: 3.0 mL SIP +6.0 mL 1X PBS
- Transfer the 4 mL of cells in 37% SIP (from step 12) into a 15 mL polypropylene conical tube.
- Slowly add 4 mL of 70% percoll, 4 mL of 30% percoll, followed by 2 mL of 1X PBS. The final solution should contain (from top to bottom): 2 mL 1X PBS, 4 mL of 30% percoll, 4 mL of cells in 37% percoll, 4 mL of 70% percoll.
Note: If processing more than 1 brain, do NOT scale up! Use multiple 15 mL polypropylene conical tubes to process 1 brain each. - Centrifuge gradient for 40 minutes at 300 x g at room temperature WITHOUT brakes.
- With a transfer pipette, remove the interphase between 1X PBS and 30% Percoll. This layer contains the myelin.
- Using another transfer pipette, remove the interphase between 70% and 37% percoll. This layer contains microglia and leukocytes. Do NOT discard! Transfer these cells to a separate 15 mL conical tube.
- Add 3X the volume of 1X PBS to the microglia cells to dilute the percoll and centrifuge for 5 minutes at 365 x g at room temperature.
- Resuspend the cell pellet in 1X PBS and proceed to cell count on the cellometer.
- Proceed to downstream cell separation, fluorescence activated cell sorting (FACS) or flow cytometry analysis (view our recommended surface and intracellular flow cytometry staining protocols) or other applications.
Note: From one adult mouse brain, a typical yield for microglia range between 200,000 – 400,000 cells using this procedure.
Representative Data
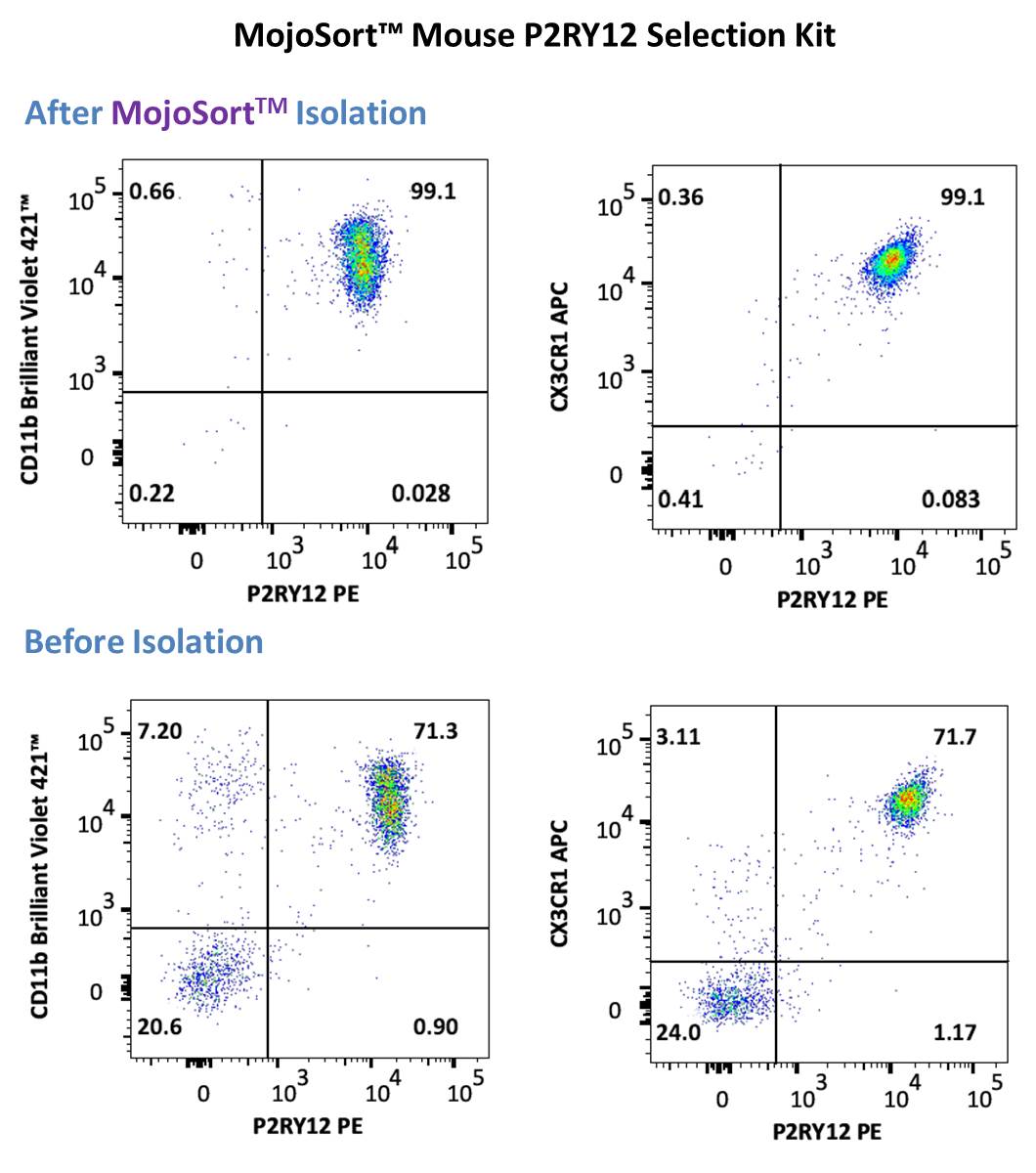
Representative data using positive selection of P2RY12+ cells using the MojoSort™ Mouse P2RY12 Selection Kit on adult C57BL/6 mouse brain prepared using brain processing, percoll gradient isolation procedure above . Cells were stained with anti-mouse P2RY12 (clone S16007D) PE and anti- mouse CD11b (clone M1/70) Brilliant Violet 421™ (left) or anti-mouse CX3CR1 (clone SA011F11) APC (right). Dead cells were excluded by 7-AAD.
Additional references: Garcia JA. et al. 2014. Curr Protoc Immunol.
 Login / Register
Login / Register 







Follow Us