Explore the Stem Cells and Development Poster
 |
| Recent advancements in stem cell research have led to important discoveries for neurodegenerative diseases, wound and tissue repair, oncology and more. We've recently updated our Stem Cells and Development Poster to include the latest in stem cell biology including development from embryonic stem cells into the mesoderm, endoderm, and ectoderm germ layers, induced pluripotent stem cells, and how the niche affects stem cell development. In this blog, we'll provide a sneak peek of the poster and further explore the concepts highlighted in the poster. Once you've finished reading, head over to the Stem Cell Poster Webpage to view products to study stem cells, download the poster, or request a physical copy to hang in the lab. |
| Embryonic Stem Cells We'll start with embryonic stem cells (ESCs) which are derived from the inner cell mass of the blastocyst and defined by both their ability to differentiate into all three germ layers, also known as pluripotency, and their ability to self-renew. One control that helps decide between these fates is regulation of the cell cycle. Stem cells that are undergoing self-renewal undergo a cell cycle characterized by short G1 and G2 phases. Alternatively, initiation of differentiation occurs during an elongated G1 phase. Many studies suggest that an interplay between cell cycle networks (including Cyclins, CDKs and Rb) and pluripotency factors drive the fate decision of whether an ESC will exit the cell cycle and differentiate or continue to self-renew (reviewed in 1). |
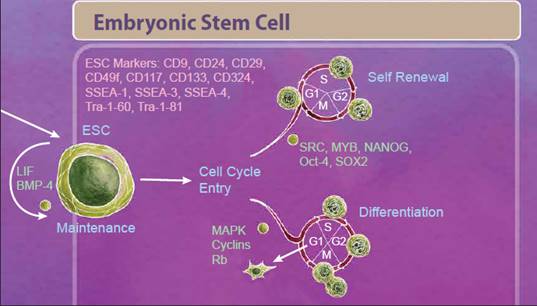 |
| Differentiating ESCs undergo reorganization and migration as part of a process known as gastrulation. During gastrulation, cells form an epiblast layer which later forms the ectoderm, endoderm, and mesoderm germ layers. From the epiblast layer, cells migrate ventrally to form the primitive streak, which establishes bilateral symmetry and eventually gives rise to both the endoderm and mesoderm germ cell layers. |
| Mesoderm Differentiation The mesoderm is derived from the middle layer of cells in the blastocyst. Organization and location of cells in the mesoderm determines the type of mesoderm that they form and ultimately what tissues they become. There are three types of mesoderm: lateral plate, intermediate, and paraxial. Intermediate mesoderm goes on to form the kidneys and the gonads. Paraxial mesoderm continues to develop into skeletal muscle, adipocytes, bone, and cartilage. The lateral plate begins to form within a cavity of the mesoderm and develops into cardiovascular tissues like cardiomyocytes, endothelial cells, and smooth muscle. Importantly, the lateral plate mesoderm also gives rise to the hemangioblast and subsequently the hematopoietic stem cell (HSC). |
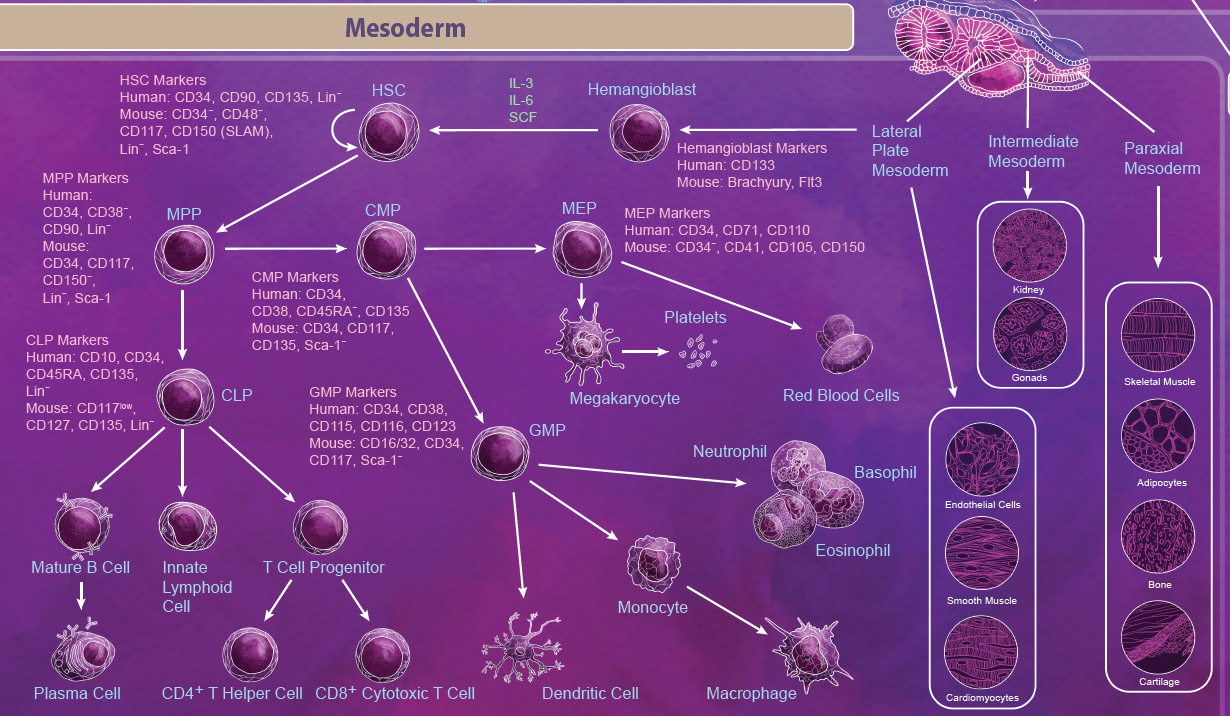 |
| The HSC is one of the most well-studied stem cells and is able to give rise to all blood cell lineages including erythrocytes, monocytes, granulocytes, megakaryocytes, and lymphocytes. First, HSCs give rise to lineage-restricted multipotent progenitor cells including the common myeloid progenitor (CMP), the megakaryocyte-erythrocyte progenitor (MEP), and the common lymphoid progenitor (CLP) which later develop into blood lineages. Like all stem cells, HSCs are able to self-renew to maintain the blood lineages throughout the body’s lifespan. In embryos, this process takes place in the yolk sac, aorta-gonad mesonephros (AGM), and the fetal liver. In adults, differentiation of HSCs occurs primarily in the bone marrow. |
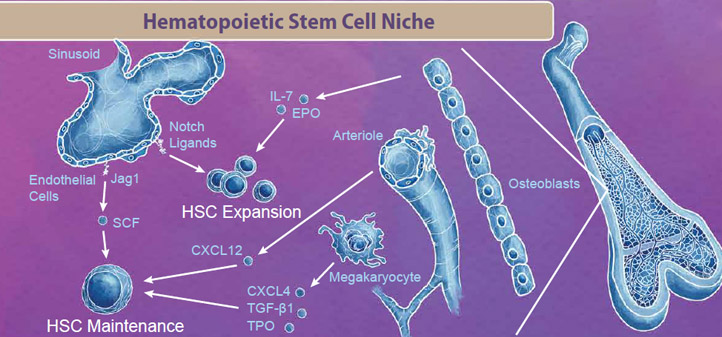 |
| The bone marrow niche contains blood cells, vasculature, bone cells, and endothelial cells that all work in tandem to provide signals for HSC maintenance and expansion. For example, endothelial cells in the sinusoid produce Notch ligands, including Jag1 and SCF, which signal to HSCs to either enter the cell cycle for either maintenance or expansion respectively. Interestingly, even cells that are originally derived from HSCs, like megakaryocytes, exist in the bone marrow niche and produce factors like CXCL4, TGF-β1, and TPO which signal to maintain the HSC pool (reviewed in 2). |
| Endoderm Differentiation | |
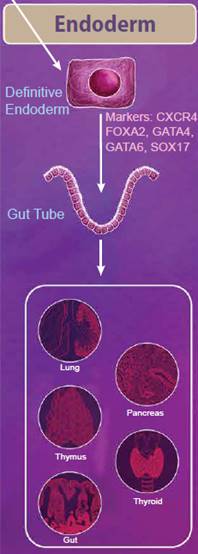 |
The endoderm is defined as the innermost germ layer and definitive endoderm cells can be identified by the expression of CXCR4, FOXA2, GATA4, GATA6, and SOX17. From the primitive streak, definitive endoderm cells egress to form a gut tube. During development, gene expression patterns change along the gut tube eventually giving rise to specific domains of cells, known as the foregut, midgut, and hindgut. Eventually, organ buds form along these domains, which develop into respiratory tissues and digestive tissues, including the lungs, thymus, pancreas, gut, and thyroid. Nodal, Wnt, and Activin signaling pathways all help to regulate these processes. While Nodal signaling is considered to be necessary and sufficient for endoderm differentiation, Activin proteins have also been used to efficiently generate definitive endoderm cells from ESCs in vitro. Wnt3a is not sufficient to generate endoderm on its own; however, it can potentiate Activin signaling to drive endoderm differentiation (reviewed in 3). |
| Ectoderm Differentiation | |
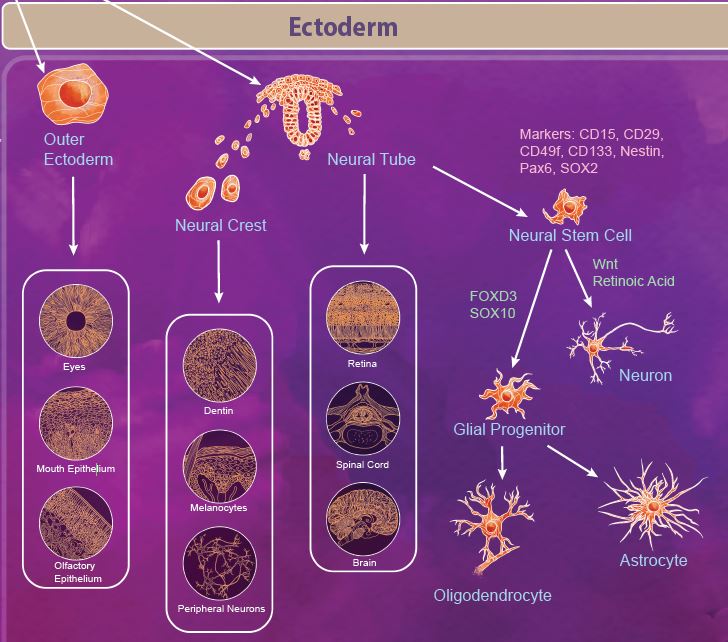 |
The ectoderm is defined as the outermost germ layer from the inner cell mass of the blastocyst. After gastrulation, the ectoderm splits into the outer ectoderm and the neural tube, in a process called neurulation. The outer ectoderm, also called the surface ectoderm, forms tissues like the olfactory and mouth epithelium and eyes. The neuroectoderm cells fold to form the neural tube from which the brain and spinal cord are formed. Additionally, there are a group of migratory cells known as neural crest cells which migrate from the neural tube to form dentin, melanocytes, and the peripheral nervous system. |
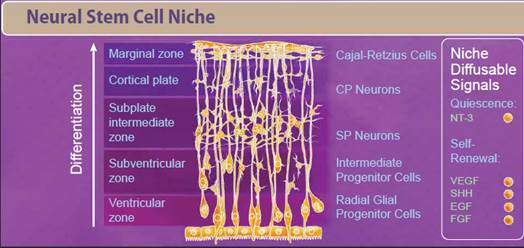
| Neural stem cells (NSCs) appear early during development and persist throughout the adult life. Like most stem cells, NSCs are heavily influenced by the niche or microenvironment from which they develop. The earliest NSCs begin in the ventricular zone where they expand, undergo asymmetrical division, and migrate basally into the subventricular zone. Here, they begin to differentiate into neurons and glial progenitor cells. This process continues as the cells migrate and differentiate through the NSC niche. Within the niche, the NSC is exposed to diffusable signals which regulate differentiation, quiescence, and self-renewal. Growth factors like EGF, FGF, and VEGF regulate self-renewal and are required for survival of the NSC population. Other signaling molecules help to regulate differentiation between neuron and glial populations, including Wnt signaling proteins, retinoic acid, FOXD3, and SOX10. |
 |
| Induced Pluripotent Stem Cells |
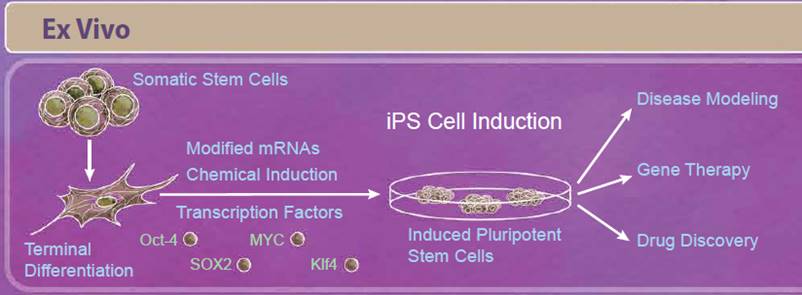 |
| Induced pluripotent stem cells, or iPS cells, are adult somatic cells that have been “de-differentiated” and resemble embryonic stem cells. First described in 2006, Yamanaka and Takahashi reported that expression of only four factors, Oct-4, SOX2, Klf4, and MYC, reprogrammed somatic cells into pluripotent stem cells that are able to differentiate into all three germ layers. Since the initial discovery, there have been improvements in reprogramming techniques including modifying both the factors used and how they are delivered to the cells. For example, some studies have found that other combinations of transcription factors may be able to induce reprogramming (including Nanog and Lin-28). Because viral transduction methods require incorporating these genes into the genome, other methods of reprogramming have also been studied including using small molecule compounds, introducing transcription factors through modified mRNAs, and CRISPR/Cas9 editing (reviewed in 5). Initially, iPS cells seemed especially promising for regenerative medicine and personalized therapies. While there are still ongoing studies in these areas, relatively few iPS cell-based therapies have moved into clinical trials so far. This is in part due to the intricacies of differentiating these cells into the tissue type of interest, verifying these cells’ function and long-term survival, and ensuring that these treatments are safe. However, in the short term, iPS cells have provided a solution for disease modeling, gene therapy, and drug discovery. Modeling certain diseases, such as neurological diseases, can be difficult due to the inability to obtain certain types of human tissues; however, iPS cells can serve as an abundant source of nearly any cell type. Because you can get a vast number of cells from a single patient, they can also serve as a source of cells for drug discovery platforms. |
| Development of diverse cell types and tissues is a complex process involving a number of signaling pathways that dictate cell fate decisions. We’ve tried to condense many of these developmental pathways in our updated Stem Cells and Development wall poster. Now that you’ve had a chance to explore the poster, don’t forget to request or download your own copy to serve as a reference whenever you need a refresher. |
| References |
|
| Contributed by Kelsey Swartz, Ph.D. |
 Login / Register
Login / Register 






Follow Us