AcuityADVANCED Protocol
Introduction
Protocol Steps
Peptide Exchange:
- ACUITYAdvanced system is recommended for use on formalin fixed paraffin embedded sections.
- Positively charged slides recommended to securely adhere tissue.
- Paraffin embedded sections must be de-paraffinized with xylene and rehydrated with a graded series of ethanol before staining.
- DO NOT let specimen or tissue dry from this point on. Optimal working dilution and incubation times are to be determined by the investigator.
Staining Protocol: Peroxidase Blocking
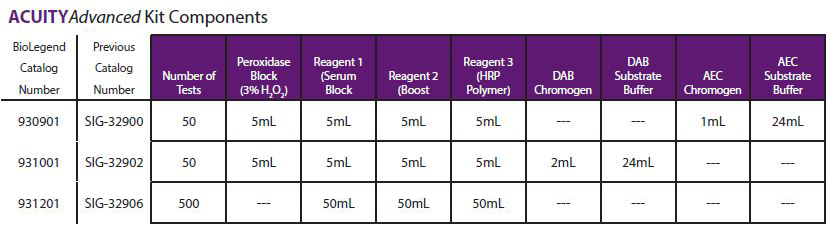
- We recommend Peroxidase Block, Catalog# 927401 or 927402. If supplied by user, prepare as per recommended protocol (supplied by user for 931201).
- When using ACUITYAdvanced hydrogen peroxide, incubate slides in 3% hydrogen peroxide blocking reagent for 10 minutes.
- Rinse with distilled water.
Heat Induced Epitope Retrieval (HIER) or enzymatic digestion
- Please refer to your antibody datasheet for recommended protocols if required.
- For HIER we recommend HIER, Catalog # 928502 (order separately). HIER or enzyme for digestion to be supplied by user.
- Wash with PBS 2 minutes, 3 times.
ACUITYAdvanced Reagent 1 (Serum Block)
- Apply 2 drops (100 µL or enough volume to cover tissue section) of ACUITYAdvanced Reagent
- Incubate in a humidity chamber for 10 minutes.
- Drain or blot off solution. Do not rinse.
Primary Antibody (supplied by user)
- Apply 2 drops (100 µL or enough volume to cover tissue section) of primary antibody.
- Incubate in a humidity chamber for 30-60 minutes.
- Rinse with PBS 2 minutes, 3 times.
ACUITYAdvanced Reagent 2 (Boost)
- Apply 2 drops (100 µL or enough volume to cover tissue section) of ACUITYAdvanced Reagent 2.
- Incubate in a humidity chamber for 15-20 minutes.
- Rinse with PBS 2 minutes, 3 times.
ACUITYAdvanced Reagent 3 (HRP Polymer)
- Apply 2 drops (100 µL or enough volume to cover tissue section) of ACUITYAdvanced Reagent 3.
- Incubate in a humidity chamber for 15 minutes.
- Rinse with PBS 2 minutes, 3 times.
Chromogen (supplied by user for 931201)
- If supplied by user; prepare as per recommended protocol.
- When using ACUITYAdvanced Chromogens (provided with kit 931001), please reference Chromogen Preparation Table.
- Rinse with distilled or tap water (AEC is alcohol soluble; do not dehydrate).
Counterstain and mount (supplied by user)
- Counterstain with desired counterstain.
- Mount and coverslip.
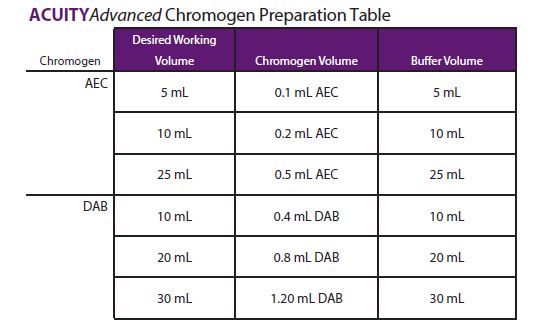
Chromogen Preparation
- AEC chromogen should be prepared 1 part AEC chromogen to 50 parts AEC Substrate Buffer.
- DAB chromogen should be prepared 1 part DAB Chromogen to 25 parts DAB Substrate Buffer. The following table provides some sample preparation examples.
Intended Use: Research Use Only (RUO); This product is sold for laboratory research use only, not for human or in vivo use.
Warranty/Conditions: BioLegend products may not be resold or modified for resale without prior written approval.
Storage: Store between 2-8°C.
General Tips & FAQ
Tips:
- Positively charged slides are recommended to securely adhere tissue.
- Paraffin-embedded sections must be de-paraffinized with xylene and rehydrated with a graded series of ethanol before staining.
- After de-paraffinization and rehydration, DO NOT let the specimen or tissue dry out.
- Optimal working dilutions and incubation times are to be determined by the investigator.
FAQ:
- For how long should I incubate with the chromagen?
- At least five minutes.
 Login / Register
Login / Register 







Follow Us