Keeping it Simple with Fluorescence
 |
Several applications utilize immunofluorescence and fluorescently conjugated antibodies. This blog focuses specifically on fluorescence microscopy, including immunohistochemistry and immunocytochemistry assays. Fluorescent detection allows you to look at multiple markers in a single sample by using discrete excitation sources for each fluorophore. This can be performed directly with a fluorophore-conjugated primary antibody or indirectly with an unconjugated primary and a fluorophore-conjugated secondary antibody. Here, we'll discuss the benefits of direct conjugates and how they can help simplify your microscopy experiments and allow you to easily expand your multicolor panels. Microscopy is commonly used when you want to visualize proteins in their native environment. As more advanced microscopy techniques are always being developed, there are many ways you can use microscopy in your research. We've listed some of the most common reasons below. |
1.) Protein-to-protein interactions can be analyzed in their native environment. This is often beneficial, as most assays tend to "mimic" the native environment of the cells of interest. This can lead to faulty conclusions and inconclusive results. 2.) Protein localization can also be determined through microscopy. This too, can play an important role, as proteins function differently in distinct parts of the cell. In addition, proteins can be quantified in those specific environments. Again, this plays an important role, especially when a treatment is applied to an organism, and understanding where that protein is increased. |
3.) Lastly, these microscopy samples can be saved for longer periods. This provides flexibility to longitudinal studies and clinical trials where researchers can re-stain samples. Given these reasons, fluorescence microscopy is both broad and flexible. |
| While there are many different forms of microscopy, fluorescence microscopy bifurcates into two categories, which are separated by their respective sample types. Samples that are derived from cells are considered immunocytochemistry (cyto=cell). Typically, these cells are either grown on or spun onto a slide and stained with either just a primary antibody or a primary and secondary antibody for signal amplification. The other major category involves a whole tissue section. These whole tissues can be sectioned at varying depths (depending on design and intention of the assay). These samples are then referred to as immunohistochemistry, as they utilize tissue sections (histo=tissue section) to study the sample of interest. |
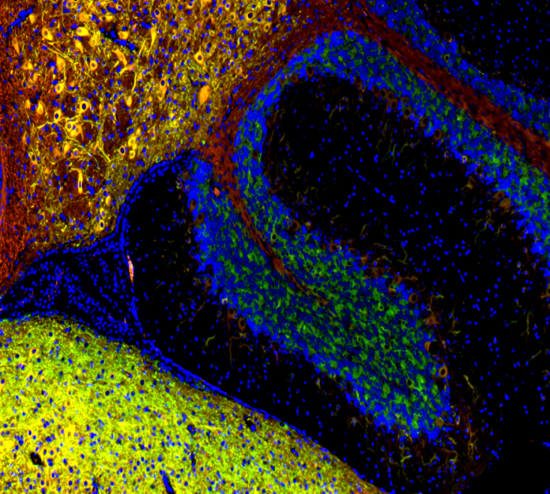 |
| Traditionally, these methodologies rely on the use of a primary and a secondary antibody. The secondary antibody will either have a fluorophore conjugated to the antibody or will use an alternative read-out method such as HRP. While the BioLegend catalog currently offers a great deal of purified antibodies for both neuroscience and cell biology applications that involve microscopy, we also have great directly conjugated primary antibodies against a variety of targets. |
While it is true that signal amplification is greatly increased when using a two-step stain, there are some advantages of using a directly conjugated primary antibody that go overlooked. 1.) Non-specific binding will always increase when you add more antibodies into an application. Antibodies display some degree of non-specific binding, regardless of specificity. If you only require one primary antibody, you might find that you produce a cleaner image. 2.) The experiment itself will be simpler and more streamlined. We all know long days in the lab do not necessarily generate effective and consistent results. Thus, with directly primary antibodies, you can help reduce experimental error and save yourself time. 3.) The use of single-step stain also makes it significantly easier to multiplex at a higher rate without worrying about whether your secondary reagent will cross-react to multiple primary antibodies that share the same isotype. This can help make your experiment more effective. 4.) Whenever our team develops a new clone and/or is interested in conjugating a new fluorophore to a primary antibody, we perform a side-by-side comparison of both the single-step stain and the two-step stain to ensure the directly conjugated primary antibody stains the target equally or brighter than the purified antibody before that antibody is placed in our catalog. |
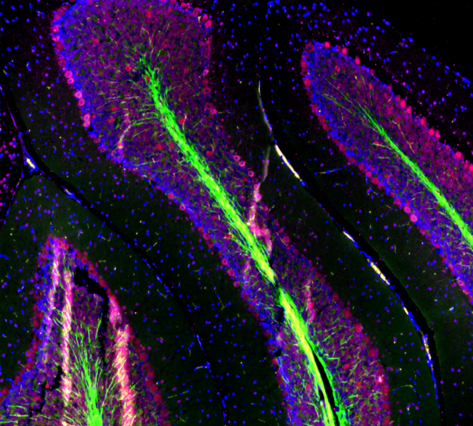 |
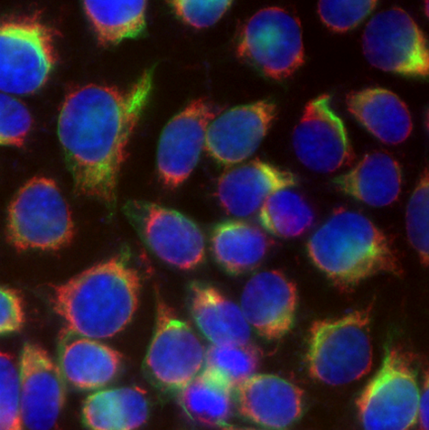 |
| Mouse brain stained with Alexa Fluor® 488 anti-Myelin Basic Protein, Biotin anti-IP3R1, Alexa Fluor® 594 anti-MECP2, and DAPI. | HeLa cells stained with Alexa Fluor® 488 Vimentin, Alexa Fluor® 594 Cytochrome C, and DAPI. |
Ultimately, the decision to use a two-step stain versus a one-step stain will always be assay-dependent as my colleague pointed out in her blog discussing primary vs secondary detection systems. For these reasons, we continue to grow our product lines for both neuroscience and cell biology targets for primary antibodies that are both affordable and effective. To learn more about direct conjugates in microscopy, check out our bio-bit. |
Contributed by Sean Cosgriff. |
 Login/Register
Login/Register 






Follow Us