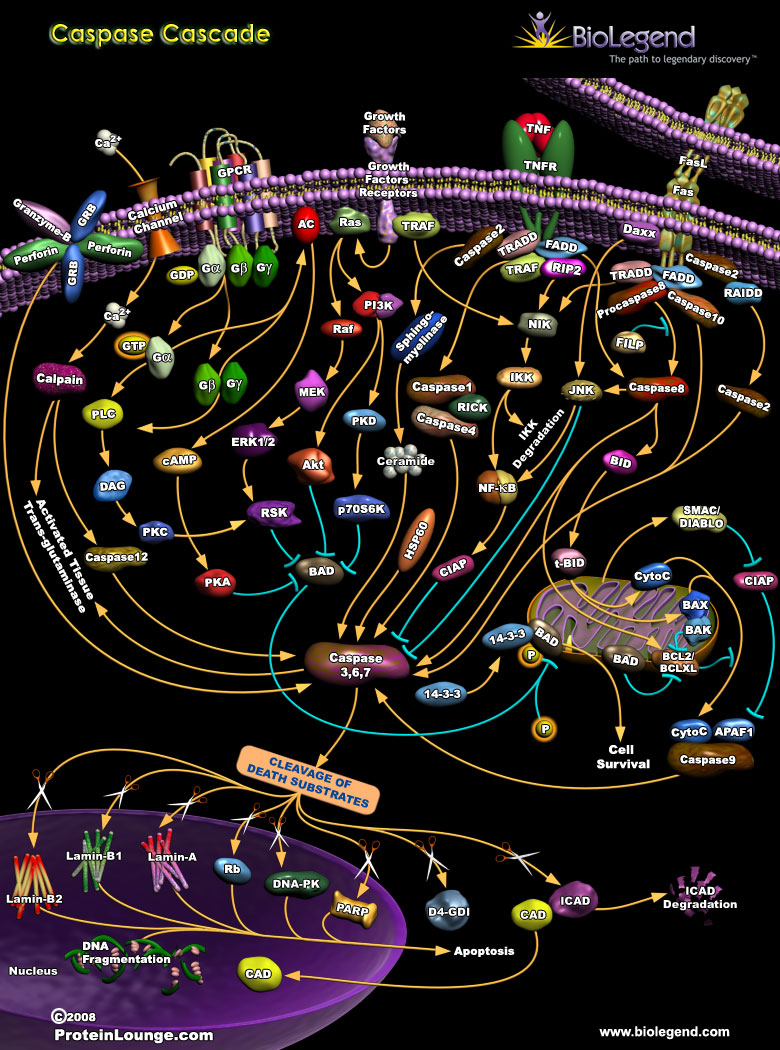
Caspase Cascade
Caspases are a group of signaling proteins that have proteolytic function, and drive apoptosis through a chain of cleavage events. There are two types of caspases: initiator caspases that are activated by dimerization, and executioner caspases that are cleaved by initiator caspases. These enzymes operate through two major signaling mechanisms, the extrinsic and intrinsic pathways. The extrinsic pathway is triggered by the binding of ligands to death receptors, which are members of the tumor necrosis factor (TNF) family of receptors including TRAIL-R1, TNFR, and Fas. Adaptor proteins like FAS-associated death domain (FADD) and TNFR-associated death domain (TRADD) then facilitate the recruitment and dimerization of procaspase-8, forming the active dimeric caspase-8. Caspase-8 goes on to cleave the executioner caspases (3, 6, 7), which will cleave substrates like Lamin and Rb to result in DNA fragmentation and apoptosis. Growth factor stimulation normally suppresses the intrinsic caspase pathway through PI3K signaling, but loss of this stimulus or the presence of other cellular stresses can activate the intrinsic caspase cascade. This involves a number of mitochondria-associated factors like BID and cytochrome C, and eventually results in the dimerization of caspase-9 and the subsequent activation of the executioner caspases.
Click on the poster below to view the interactive version.
 Login / Register
Login / Register 







Follow Us