Primary vs Secondary Detection Systems
 |
What is a Secondary? |
| A secondary reagent is used to identify or enable the detection of a primary antibody or reagent. Primary reagents are antibodies specific to the target or cell marker of interest. If the primary antibody is unlabeled, a secondary reagent that is labeled would be required to bind to the primary reagent on the cell and enable its detection. While many secondary detection systems use a purified antibody with an anti-Ig secondary antibody, biotin-labeled antibodies and conjugated streptavidin molecules can also be used. Biotin is a water-soluble form of vitamin B that is used as a cofactor for a variety of metabolic processes. Streptavidin is a bacteria-derived protein with an extremely high affinity for biotin (Kd of approximately 10-14). These two molecules bind together almost irreversibly, making them ideal candidates for secondary detection. |
Advantages of Using Secondary Reagents |
| There are 3 main reasons why you would want to choose a secondary labeling system for your experiment. |
1. Restrictions on Formats of Primary Antibody Available Sometimes for studies, researchers will need to have an antibody in a specific fluor or format that may not be commercially available. It can be easier to find a purified or biotinylated version of the primary antibody of choice. In contrast, secondary reagents are very flexible and are often available in a wider variety of formats than primary antibodies. |
2. Expression Levels of Target are Low Using a secondary reagent for detection often leads to signal amplification that can make low-expressing targets easier to detect. Many secondary reagents are conjugated at similar fluorophore to protein ratios as primary antibodies, but it is common for multiple secondary antibodies to bind to a single primary antibody, giving an overall stronger signal. |
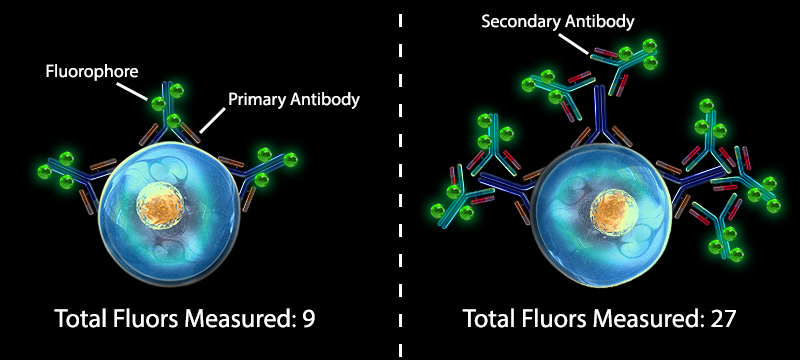 Comparing the signal detection of a primary labeling system to signal from a primary + secondary labeling system |
| 3. Reagent Flexibility Incorporating a secondary reagent can allow a single primary antibody to be used in multiple applications. For example, if you have an antibody that works for western blotting and microscopy, utilizing secondary reagents can allow you to use this clone for both applications. Secondary reagents are often more economical than buying additional directly conjugated antibodies and can add flexibility in panel selection for flow cytometry. For example, purchasing biotinylated lineage antibodies can allow you to easily adjust the color of your lineage cocktail by simply swapping out the streptavidin used to label the lineage markers rather than replacing the entire cocktail. |
How to Choose the Correct Secondary Reagent |
| There’s a few factors that need to be taken into consideration when choosing the best secondary reagent for your experiment. |
1. Conjugation It's important to verify that the secondary antibody has the proper conjugation for your experiment. If you are doing a western blot, an HRP conjugate can be used with a substrate solution, whereas a fluorophore-conjugated secondary is more appropriate for use in flow cytometry. While the majority of our fluorophores can be used for flow, we recommend using the Alexa Fluor® dyes, Brilliant Violet™ 421, and Brilliant Violet™ 510 for microscopy applications. Check out our microscopy page to see how these products perform in ICC, IHC-F, and IHC-P. |
2. Primary Antibody Host Species & Sample Type The most commonly used secondary reagents are antibodies that are raised against the immunoglobulins of another species, so you will need to check the host species and isotype of your primary antibody to ensure your secondary will recognize the primary antibody. You'll also need to keep your sample type in mind; for example, using an anti-mouse secondary on mouse samples may result in high background as the secondary will recognize both the primary antibody of interest as well as any endogenous immunoglobulins that may be present in the sample. |
3. Staining Panel Composition When staining with multiple primary antibodies, it is important to ensure that your secondary reagent will only bind with one primary antibody of interest. This can sometimes be difficult to do as antibody availability can be limited, so consider using a biotin-labeled primary with a streptavidin-labeled secondary to help mitigate this issue. For larger multicolor panels, it may be necessary to use directly conjugated primary antibodies instead of secondary antibodies. |
4. Assay Adjustments Utilizing secondary reagents can also impact how you design your overall experiment. Incorporating secondary reagents can sometimes lead to increased non-specific binding and background noise. As such, it is important to include a secondary-only stained sample as a control. Additional blocking steps may also be needed. For secondary antibodies, blocking with a buffer containing serum that matches the species of the secondary can help reduce non-specific binding to the surface of your cells. Using streptavidin secondaries may require a protocol to block or neutralize any endogenous biotin that may be present in your sample. |
| Still not sure if you should use a direct conjugate or secondary system? Check out our secondary reagents or email us at tech@biolegend.com and we'll help you choose! |
| Contributed by Samantha Stanley, PhD. |
 Login / Register
Login / Register 






Follow Us