Tau is a family of neuronal proteins that bind to microtubules (the neuron's transport system), and stabilize their formation and maintenance. In the human brain, Tau proteins constitute a family of 6 isoforms that is produced by alternative splicing of a single gene called MAPT (Microtubule-Associated Protein Tau). Research interest in tau proteins began to grow when tangled forms of these proteins were found to make up the paired helical filaments in brains of Alzheimer’s disease (AD) patients.
Tau Proteins
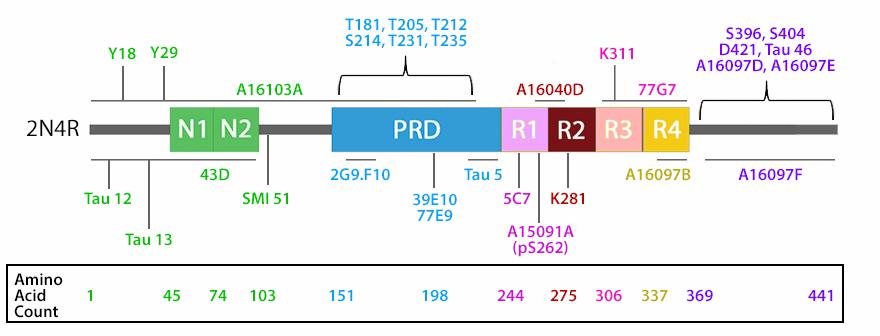
Several phosphorylation sites have been identified in tau proteins. Tau phosphorylation at different sites has a different impact on its biological function and on its pathogenic role. The normal level of tau phosphorylation is a consequence of dynamic regulation of tau kinases and tau phosphatases. The major tau kinases include glycogen-synthase kinase-3β (GSK-3β), cyclin-dependent protein kinase 5 (cdk5), cAMP-dependent protein kinase (PKA), and stress-activated protein kinases. Among the protein phosphatases, PP2A is known to be an important one. Despite extensive studies, the causes leading to abnormal hyperphosphorylation of tau are still not fully understood. The hyperphosphorylation of the tau protein reduces its binding affinity to microtubules, thus disrupting the structural organization and maintenance. Excess levels of unbound tau protein leads to the formation of tau aggregates, insoluble fibrils, and intracellular neurofibrillary tangles observed in Alzheimer's disease (AD) and other tauopathies. In addition to hyperphosphorylation, alterations of tau itself, such as mutations in the MAPT gene, also plays a role in its aggregation.
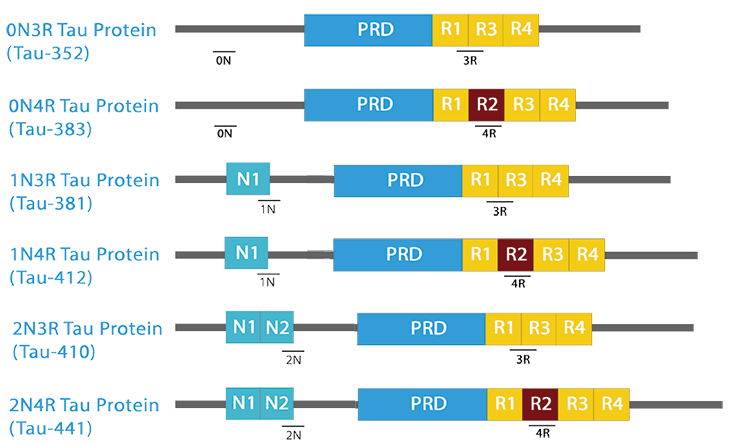
The isoforms of the tau proteins are the products of alternative splicing of exons 2, 3, and 10 of a single gene that in humans is known as MAPT (Microtubule-Associated Protein Tau). The six tau isoforms are designated as 352, 381, 383, 410, 412 and 441. They differ from each other by the presence or absence of one or two inserts (N1, N2) in the N-terminal part, and by the presence of either three repeats (R1, R3, R4) or four repeats (R1, R2, R3 and R4) in the C-terminal part. The repeat domains, located in the C-terminus of Tau, are believed to be important for microtubule binding as well as for the aggregation of tau into paired helical filaments.
| The isoforms of the tau proteins are the products of alternative splicing of exons 2, 3, and 10 of a single gene that in humans is known as MAPT (Microtubule-Associated Protein Tau). The six tau isoforms are designated as 352, 381, 383, 410, 412 and 441. They differ from each other by the presence or absence of one or two inserts (N1, N2) in the N-terminal part, and by the presence of either three repeats (R1, R3, R4) or four repeats (R1, R2, R3 and R4) in the C-terminal part. The repeat domains, located in the C-terminus of Tau, are believed to be important for microtubule binding as well as for the aggregation of tau into paired helical filaments. |
 |
Isoform Specific Antibodies: Tau 0N,
3H6.H7 Tau 4R,
5F9 Tau 2N,
71C11 |
 Login/Register
Login/Register 






Follow Us