Rule the Magnet! Tips on improving your magnetic cell separation results
| Our MojoSort™ magnetic cell separation reagents, as well as others in the market, offer convenient, efficient, and cost-effective ways to obtain purified cells of interest. But, as with any other procedure, you may run into some difficulty with your separation experiment. Perhaps your cells are not as pure as you'd want/expect, and you don't know how to get rid of the cells you don't want? Or, maybe the resulting number of cells you get out in the end (i.e. yield) is not very high, as if you're somehow losing a lot of cells in the process? In this blog, we'll discuss some of the common issues that can yield suboptimal results from a magnetic separation experiment, how to overcome them, and how you can bring some mojo back to your experiment! |
|
Types of MojoSort™ Products |
| Positive vs. Negative Selection: After positive selection, the final cells of interest will be bound to the magnet. Our "Selection Kits" include cell type-specific antibodies, along with nanobead-conjugated secondary reagents (e.g. Streptavidin-nanobeads) to purify the cells of interest as advertised. You can find an example of one of our selection kits with the MojoSort™ Human CD14 Selection Kit. In a negative selection (or isolation) experiment, the cells of interest are not bound by the magnet and are ultimately eluted out in the negative fraction. All other unwanted cells are depleted out in the magnetic-bound fraction via the use of antibody-nanobead cocktails. One major advantage of using negative selection is the cells of interest in the end are not bound to magnetic particles. This may be preferable if there are any concerns about the interference of magnetic particles in a sensitive downstream bioassay or application. The difficulty in designing this on your own is that you must be able to efficiently deplete all other cells in a given sample in order to get good purity, which might be difficult to do comprehensively without trial and error. Our isolation kits are optimized to deplete out all other cells in a given sample except for the cells advertised. For instance, our MojoSort™ Human NK Cell Isolation Kit contains a cocktail of antibodies to deplete out all other cells from healthy PBMC samples except NK cells, as analyzed by CD56+CD3- surface marker phenotype shown on the product webpage. Direct vs. two-step isolation: Direct separation refers to the use of magnetic particles that are directly conjugated to the antibodies. Two-step utilizes antigen-specific primary antibodies, followed by Streptavidin-magnetic particles (in the case where antibodies are biotinylated), or other magnetically-bound secondary reagents, such as our anti-PE and anti-APC nanobeads. MojoSort™ products that are labeled as "nanobeads" contain antibodies that are directly conjugated to magnetic particles, and our "selection kits" or "isolation kits" contains reagents for a two-step selection method. |
| So, how can you break down your result and make improvements to your experiment? Identify the perpetrator. With low purity, the source of "contamination" is likely originating from one cell population. Try and identify this population. When assessing purity via flow, analyze the plots using multiple markers and parameters instead of just looking at a marker associated with the cells of interest in a histogram. Knowledge of the starting cell populations/contents is useful here, as is having a good set of controls to identify them. If you are using a "home-made" magnetic depletion cocktail instead of a pre-optimized kit from manufacturers, honing into particular cell populations allow you to pinpoint where you need to optimize your experiment, such as adding markers specifically for those cells to better deplete, and titrating associated reagents. A couple of additional tips here: |
|
|
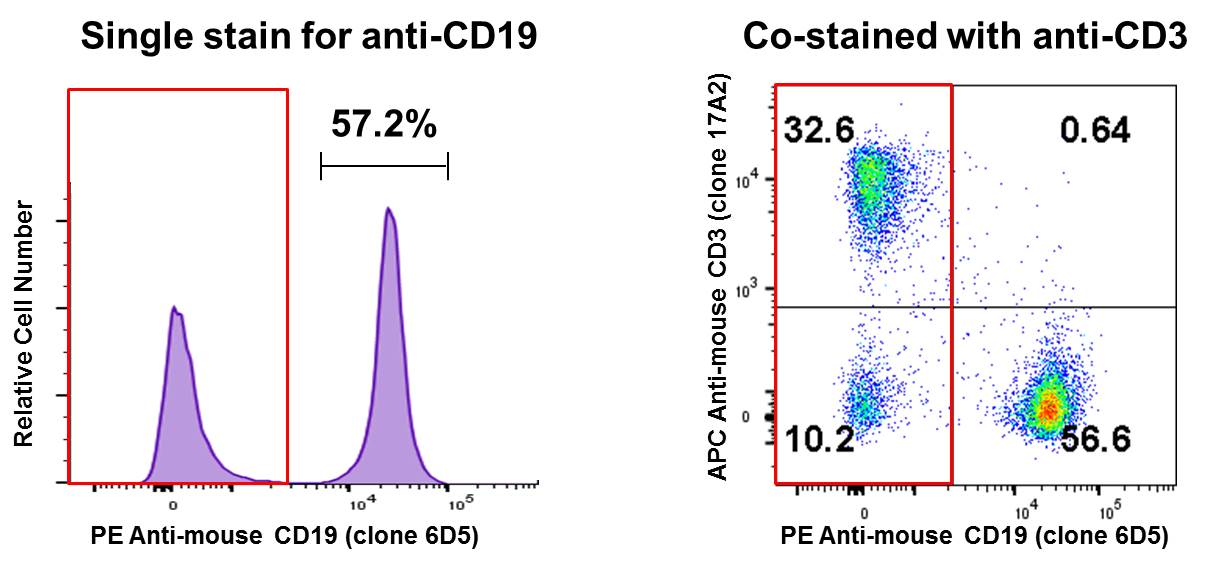
Co-staining a sample and analyzing on a two-dimensional bivariate plot instead of a histogram can reveal a lot of information. In the B Cell isolation example above, co-staining with APC anti-mouse CD3 suggests that the remaining CD19- population (red box) is primarily T cells (~32%). Perhaps this is where you can optimize your experiment, to try and remove more T cells from your sample: |
 |
| The cells are too concentrated in the separation experiment A common issue leading to poor magnetic cell separation is a staining reaction with a sample that is too highly concentrated with cells. I know that sometimes it's cumbersome to count out the cells you have in your sample, and just taking 100 µL straight out of your original sample suspension allows you to cut so much time and hassle! However, this step is crucial - if your sample is too concentrated, it can affect antibody (and any other secondary reagent) binding its target antigen sufficiently and/or increase non-specific binding, causing more cell aggregation, amongst other unwanted consequences. Ultimately, this can affect both purity and yield. If using a column, applying too many cells per run can also clog it. Make sure you are following manufacturers instructions on how many cells can be subjected to each cell separation assay. Also verify the column manufacturer's recommendation so samples are diluted to a sufficient volume before they're added to the columns, and pre-equilibrate columns with proper buffers as necessary. For most MojoSort™ reagents, a standard test consists of 1 x 10^7 cells in a 100 µL reaction volume. You can always scale up as needed, but try to always keep the same cell/volume ratio. When starting with less than 10^7 cells, I'd recommend staying with the 100 µL reaction volume. Manual cell counting can yield variability, so make sure you have a relatively precise number. If you're concerned about precision, try counting a couple of replicates. |
| Don't get too physical Take your time, and be gentle. If using our MojoSort™ magnetic separator (or equivalent), if you rotate the tube while inside the magnet during incubation, it can cause the magnetically-bound cells to come loose from the magnet. So, during this incubation step, leave the tube alone. Don't aggressively shake the tube to get your unbound samples out- use a smooth, decanting motion to take out your negative fraction. If using a column, let gravity do the work and allow time for samples to naturally drip through. Don't plunge the bound samples out until all of the negative fraction has dripped through. |
| Low yield? Labeling too much... or too little? The likely cause for your yield being lower than expected is that your cells are ending up in the wrong fraction. In a positive selection experiment, the cells are likely being washed away. For negative isolation, you're depleting out too many of your cells of interest. You can confirm this by taking both positive/negative fractions for flow analysis, but in either case, the first thing you can try is to optimize reagent use: |
|
|
For MojoSort™ reagents, contact our tech support team (tech@biolegend.com) to get sample yields obtained from our in-house testing. Also note that increasing yield can potentially concomitantly compromise purity. The key here is to find a "sweet-spot" that has a good balance of both. |
| Follow recommended protocol! This one's relatively straightforward - if you are using one of our pre-designed kits, we’ve developed optimized protocols, which are available on their respective product webpages or you can view our Protocol tables on our MojorSort™ page. It's best to start with what's been validated to work in BioLegend labs first, before proceeding to optimize and modify steps and reagents as desired in your future experiments. To see how our MojoSort™ reagents and magnets are used, check out our awesome protocol video below: |
 |
| The points mentioned here are by no means comprehensive as there are numerous other factors and considerations not covered here that can affect your magnetic separation outcome. However, if you encounter low purity and/or yield, hopefully some of the tips and suggestions above may help as your first steps to success. You can check out the MojoSort™ webpage for an overview on our reagents, FAQs, and protocols by clicking on the various available tabs. For any additional suggestions on how to design and improve your magnetic separation experiments, contact us at tech@biolegend.com! |
| Contributed by Kenta Yamamoto, PhD. |
 Login/Register
Login/Register 






Follow Us