Uncover the Layers of Parkinson’s Pathology
 |
Parkinson’s disease (PD) is defined by the death of dopamine-producing (dopaminergic) neurons in the substantia nigra, a brain region important for controlling movement. This type of neuronal loss, which can be caused by failure of neuroprotective mechanisms, results in motor dysfunctions like muscle stiffness, slowness, and uncontrolled tremors – symptoms used for the clinical diagnosis of PD. Currently there are no biomarkers or brain imaging methods that can confirm PD in a patient, though post-mortem analyses have identified histopathological hallmarks of disease. One such hallmark is the presence of inclusions called Lewy bodies, aggregates of proteins and cellular components that are associated with multiple brain disorders, including PD and certain types of dementias. A better understanding of Lewy body composition could help us develop more advanced diagnostic tools and identify potential therapeutic targets. In this blog post, we highlight a recent study that uses high-resolution microscopy to describe the onion-like architecture of Lewy bodies. Could these new insights change how we view PD pathology? |
| Lewy Body Layers Alpha-Synuclein, a protein involved in synaptic function in the brain, is known to be a major constituent of Lewy bodies. Two post-translationally modified (PTM) variants of α-Synuclein are increased in abundance during PD: a shortened form of the protein that is truncated at the C-terminus (CTT-122), and a full-length protein that is phosphorylated at serine 129 (pS129). Previous work has provided us with a basic picture of CTT-122 and pS129 within Lewy bodies, but the specific roles of these variants in Lewy body formation are not completely understood. |
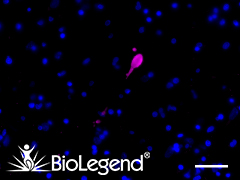 IHC staining with purified anti-α-Synuclein, aggregated antibody (clone A17183G) on formalin-fixed paraffin-embedded Parkinson’s disease brain tissue. Following staining with primary antibody overnight at 4°C, tissues were incubated with Alexa Fluor® 647 goat anti-rat IgG (Cat. No. 405416) for one hour at room temperature. Nuclei were counterstained with DAPI. We are currently offering a 50% discount on anti-α-Synuclein antibodies; see list here. |
 |
A study by Moors et al. (available in preprint on bioRxiv) utilized immunofluorescent staining combined with advanced microscopy methods, including stimulated emission depletion (STED), to map the distribution of these α-Synuclein variants in brain tissues. STED microscopy dims surrounding fluorescence to illuminate a focal point, which enabled the researchers to capture super-resolution images of CTT-122 and pS129 localization. CTT-122 was found to co-localize with mitochondria in both healthy and diseased brains, while pS129 was strongly associated with pathology. Within Lewy bodies, CTT-122 was grouped with proteins and lipids at the core, and this was surrounded by outer layers of neurofilament-embedded pS129. By identifying patterns of α-Synuclein distribution, the study authors were able to elaborate on a working model of Lewy body maturation. They propose that the failure of protein degradation machinery leads to the accumulation of pS129 aggregates, which interact with neurofilaments to induce compaction of Lewy bodies into onion-like structures. The researchers suggest that their findings support a central role for pS129 in driving the formation of these “onions”, which could inform future approaches for using α-Synuclein as a PD biomarker or therapeutic target. |
| PTM-Specific Antibodies Research by Moors et al. relied heavily on the use of anti-α-Synuclein antibodies that could bind specifically to different variants of the protein. As we learn more about the role of α-Synuclein in PD pathology, researchers will need antibodies that can reliably discriminate between various PTMs, including forms of the protein that have yet to be discovered. To help with this important research, we partner with leaders in the PD field, like The Michael J. Fox Foundation, to create novel reagents and solutions that overcome research hurdles. In honor of Parkinson’s Awareness Month, we are offering a 50% discount (until May 31, 2020) on our anti-α-Synuclein antibodies that can be used to detect phosphorylated, truncated, and aggregated forms of the protein: |
| Specificity | Clone | Reactivity | Applications |
|---|---|---|---|
| α-Synuclein (C-Terminal Truncated x-122) |
A15127A | Human | IHC-P, ELISA |
| α-Synuclein, 117-122 | A15126D | Human | IHC-P, WB, ELISA |
| α-Synuclein, 34-45 | A15110D | Human | IHC-P, ELISA |
| α-Synuclein, 80-96 | A15115A | Human, Mouse, Rat | IHC-P, WB, ELISA |
| α-Synuclein, aggregated | A17183A | Human | IHC-P |
| α-Synuclein, aggregated | A17183B | Human | IHC-P |
| α-Synuclein, aggregated | A17183G | Human | IHC-P |
| α-Synuclein Phospho (Ser129) | P-syn/81A | Human | IHC-P, ICC, WB |
(Promotional pricing cannot be combined with other discounts. Price reduction will be reflected during checkout. If you are a customer who orders via a distributor, please contact your distributor for eligibility.) Learn about other discounted antibodies for Parkinsons’ research Learn more about ‘Neuroprotection in the Parkinson’s Brain’ by checking out our other blog post. Interested in receiving more related content? Sign up for our email updates. |
References:
|






Follow Us