| At tradeshows, it’s common for our booth visitors to fawn over our newest posters and take several of them home. While it might only take you a few seconds to hang, did you know that each poster takes roughly 6-9 months to complete? A lot of fact-checking, sketching, graphic design, and effort goes into making these posters, so we wanted to start to dive into them to show you what they offer. Our first in-depth look is with our new Innate Immune Signaling Poster. Each pathway’s arrows have been separated by easy-to-identify colors to avoid confusion. Arrows were left white if multiple pathways converged on a molecule. If you want to follow along, explore with our Interactive Poster, which contains links to relevant products. You can also request the free poster here. |
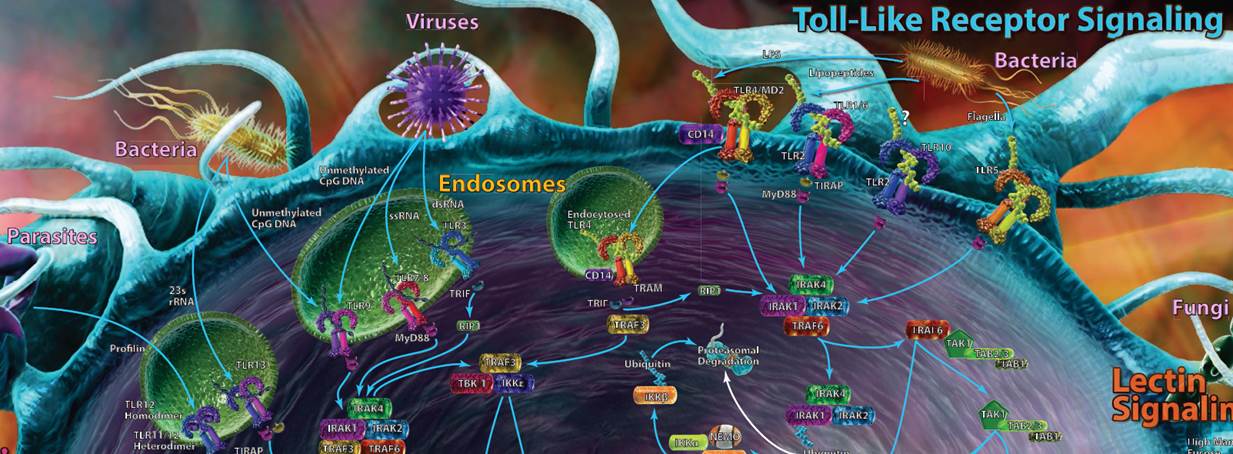 |
| Toll-Like Receptor Signaling |
| Toll-like receptors (TLRs) are most likely the first set of pathways that you recognized. TLRs can be found on a variety of cells and work as pattern recognition receptors, picking up markers that are typically highly conserved among bacteria, viruses, fungi, and protozoa. Some TLRs are present on the cell’s surface, while others are buried within endosomes to help combat internal pathogens like viruses. |
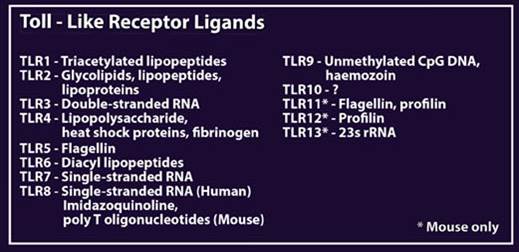
| Recognition of these foreign markers triggers a cascade of innate immune responses, including cytokine and interferon production and inflammation. Most of the TLR pathways take advantage of at least one adaptor protein for downstream signaling, including MyD88, TIRAP, TRIF, or TRAM. In humans, 10 functional TLRs have been discovered, while in mice, up to 13 have been discovered. The ligands for each can be seen in the table at the bottom of the poster. |
 |
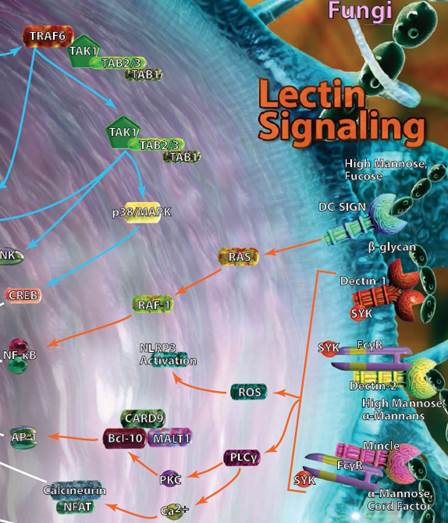
| Lectin Signaling |
| A select group of C-type lectin receptors (CLRs) are classified as pattern recognition receptors and can bind carbohydrate structures that can be found on various bacteria, viruses, or fungi. DC-SIGN, Dectin-1, Dectin-2, and Mincle are among the most well-known receptors in this group and can typically be found on monocytes, macrophages, and dendritic cells. Activation of these pathways leads to the production of several classical inflammatory mediators like NF-κB, and the release of cytokines associated with both the innate and adaptive immune response. |
 |
| |
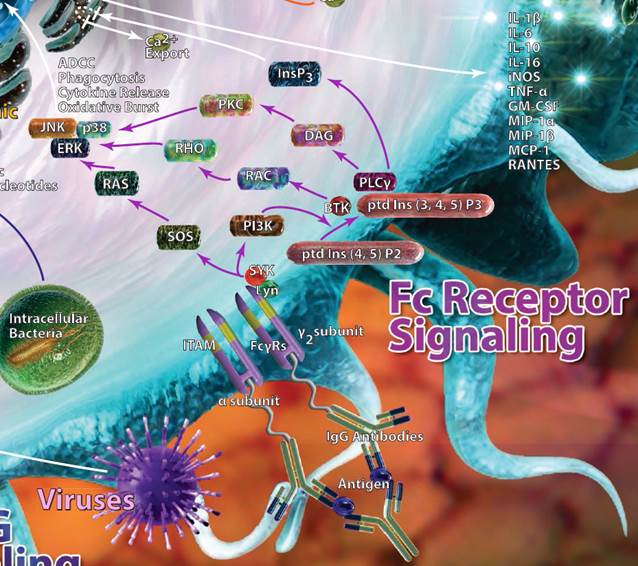
| Fc Receptor Signaling |
| As their name implies, Fc receptors are designed to pick up the Fc (fragment, crystallizable) portions of antibodies. They can be found on B cells, dendritic cells, NK cells, macrophages, and granulocytes. Different Fc receptors may bind to different antibodies, as denoted by the greek letter that follows their name. For example, FcγR recognizes IgG antibodies, whereas FcεR would recognize IgE antibodies. Binding of antibodies by the Fc receptors triggers a variety of effects, including phagocytosis and cytokine, histamine, or enzyme release. |
 |
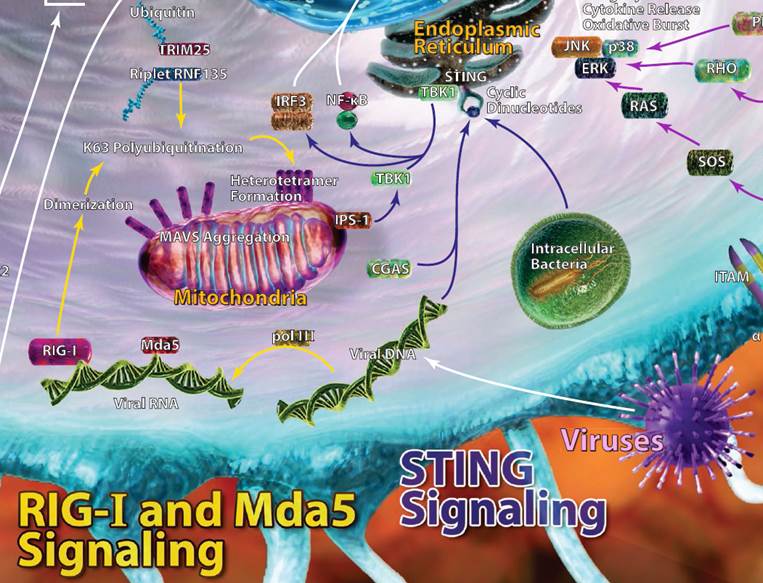
| Rig-I, MDA5, and STING Signaling |
| Viral RNA is often injected into host cells and requires replication and transcription in order to propagate new viral RNA strands.This newly synthesized viral RNA can be recognized by dsRNA helicase enzymes like retinoic acid-inducible gene 1 (Rig-I) and fellow RIG-I-like receptor family member MDA5. More specifically, RIG-I recognizes 5’ phosphorylated blunt ends of viral genomic dsRNA. MDA5 also binds viral RNA, but doesn’t have a 5’ or 3’ end it favors. Upon binding, both RIG-I and MDA5 eventually recruit a signaling adaptor, MAVS. This leads to production of type I interferons and other inflammatory mediators to help clear viral infections. Working in a similar capacity, stimulator of interferon genes (STING) is a protein that can recognize dsDNA and cyclic dinucleotides. STING can typically be found on endoplasmic reticulum and interacts with TBK1, leading to IRF3 activation and type I interferon production. Autophagy can also be induced via STING. |
 |











Follow Us