Research that reveals a better understanding of neurological systems, and the diseases that affect them, could translate into cures that improve millions of lives. BioLegend offers a wide-ranging portfolio of products, providing solutions for your neuroscience experiments, including antibodies, immunoassay solutions, recombinant proteins, magnetic cell separation, and single-cell proteogenomics reagents. Our reagents were built in collaboration with preeminent leaders in the field, like The Michael J. Fox Foundation (see the Product Highlights tab below), signifying our commitment to partner with your teams to seek out new discoveries, bringing our customers new choices and comprehensive solutions for studying neurological disorders.
Contact your local technical representative to collaborate, partner, and discover new opportunities.
 Login / Register
Login / Register 



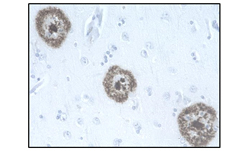 Neurodegeneration is a complex biological process that is often defined by the presence of protein aggregates. Protein aggregation is a result of misfolded proteins forming fibrils, inclusion bodies, and other large proteinaceous inclusions such as plaques. Many neurodegenerative disorders are associated with aggregation of key target proteins including Amyloid-β (Aβ) and Tau in Alzheimer's disease, and α-Synuclein in Parkinson's disease. Protein aggregates exert their toxic function by disrupting normal cellular and synaptic functions ultimately resulting in cell death. We provide a large assortment of antibodies, kits, and recombinant proteins to study the function of these targets in a neurological setting.
Neurodegeneration is a complex biological process that is often defined by the presence of protein aggregates. Protein aggregation is a result of misfolded proteins forming fibrils, inclusion bodies, and other large proteinaceous inclusions such as plaques. Many neurodegenerative disorders are associated with aggregation of key target proteins including Amyloid-β (Aβ) and Tau in Alzheimer's disease, and α-Synuclein in Parkinson's disease. Protein aggregates exert their toxic function by disrupting normal cellular and synaptic functions ultimately resulting in cell death. We provide a large assortment of antibodies, kits, and recombinant proteins to study the function of these targets in a neurological setting.


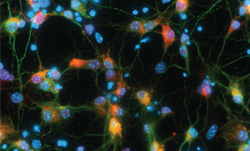

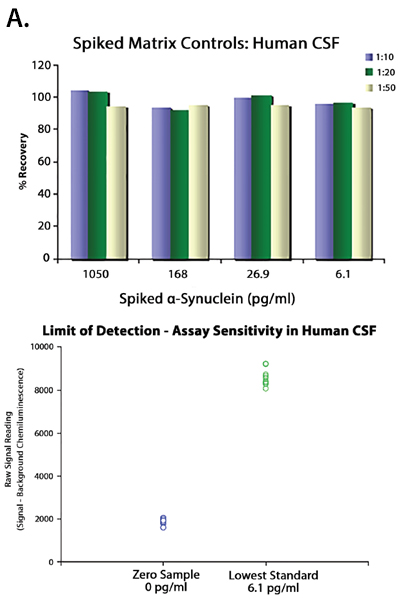
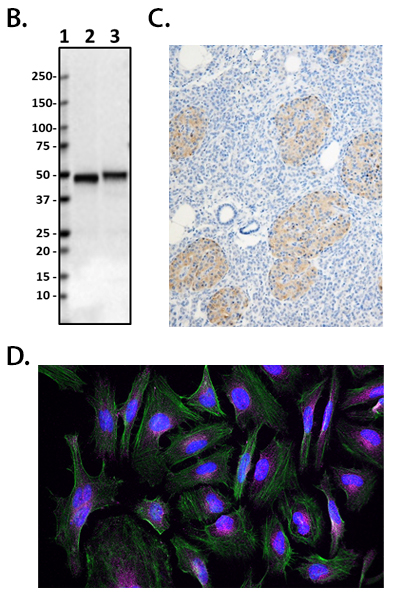
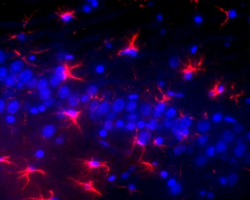
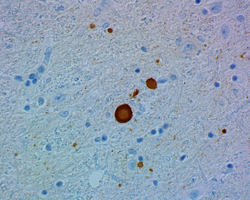 Having reagents that can differentiate between aggregated and non-aggregated forms of proteins is vital in researching neuropathies. To this end, we’ve created a number of in-house clones that recognize aggregated α-Synuclein (clones
Having reagents that can differentiate between aggregated and non-aggregated forms of proteins is vital in researching neuropathies. To this end, we’ve created a number of in-house clones that recognize aggregated α-Synuclein (clones 



Follow Us