 |
| Protein aggregation is a hallmark of a number of neurodegenerative diseases. While different diseases are associated with the accumulation of distinct proteins, the process of aggregation from the formation of oligomers to protofibrils to mature aggregates is similar. In a recent review, Soto and Pritzkow discuss these similarities and the process by which protein aggregates are transmissible. Transmission of protein aggregates can be described using the seeding‑nucleation model in which pre‑formed aggregates or “seeds” recruit normal proteins into the aggregate. Aggregates can also undergo fragmentation releasing more seeds which can spread from cell‑to‑cell or brain region‑to‑region, similar to the process of Prion infection. To study the process of protein aggregation in neurodegeneration, BioLegend offers several clones that are specific to aggregate or fibril forms of proteins associated with neurodegenerative diseases including β‑Amyloid and α‑Synuclein. |
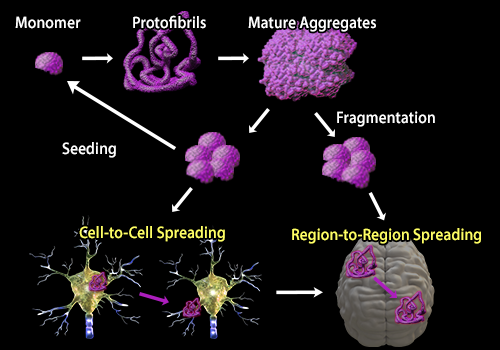 |
| Adapted from Soto and Prtizkow, 2018. Nature Neuroscience. 21‑1332‑1340. Pubmed. |
 |
|
Learn more about our new β‑Amyloid aggregate‑preferring clone, 3A1! |
 |
*Any references to promotions on this page may not be valid at this time. View our promotions page for the most up-to-date promotions.
 Login / Register
Login / Register 







Follow Us