MojoSort™ Human CD14+ Monocyte Isolation Kit Column Protocol
Introduction
BioLegend MojoSort™ nanobeads work in commonly used separation columns, based on our internal research as well as validation by external testing by academic labs. This simple protocol consists of following the MojoSort™ protocol to label the cells with pre-diluted MojoSort™ reagents and using the columns as indicated by the manufacturer.
Note: Due to the properties of our beads, it may be possible to use far fewer beads and less antibody cocktail that with other commercial suppliers. We recommend a titration to find the best dilution factor. However, as a general rule, dilutions ranging from 1:2 to 1:10 for the antibody cocktail can be used. Dilutions ranging from 1:5 to 1:20 for the Streptavidin Nanobeads can be used. Please contact BioLegend Technical Service (tech@biolegend.com) if further assistance is needed.
Important Note
MojoSort™ magnetic particles can be used with other commercially available magnetic separators, both free standing magnets and column-based systems. Because MojoSort™ protocols are optimized for the MojoSort™ separator, the protocols may need to be adjusted for other systems. Please contact BioLegend Technical Service (tech@biolegend.com) for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Sample Preparation: It is strongly recommended that platelets be removed prior to the isolation of monocytes using a suitable method. See recommended platelet removal protocol below.
Protocol Steps
Platelet Removal Protocol
- Dilute blood with 2-4 times (volume/volume) 1X PBS.
- Carefully layer diluted blood over 12.5 mL of isolation medium in a 50mL tube.
- Centrifuge at 400xg for 25 minutes at room temperature in a swinging-bucket rotor without the brake.
- Aspirate the upper layer of the gradient (serum), leaving the interphase containing the mononuclear cells undisturbed.
- Carefully transfer the mononuclear cells to a new 50 mL tube.
- Fill the tube with 1X PBS, mix, and centrifuge at 200xg for 8 minutes at room temperature. Carefully remove supernatant as much as possible.
- Repeat step 6.
- Proceed to separation protocol.
Separation Protocol
- In the final wash of your sample preparation, resuspend the cells in MojoSort™ Buffer by adding up to 4 mL in a 5 mL (12 x 75 mm) polypropylene tube.
Note: Keep MojoSort™ Buffer on ice throughout the procedure. - Filter the cells with a 70 µm cell strainer, centrifuge at 300xg for 5 minutes, and resuspend in an appropriate volume of MojoSort™ Buffer. Count and adjust the cell concentration to 1 x 108 cells/mL.
- Aliquot 100 µL of cell suspension (107 cells) into a new tube. Add 5 µL of Human TruStain FcX™ (Fc Receptor Blocking Solution), mix well and incubate at room temperature for 10 minutes. Scale up the volume accordingly if separating more cells. For example, if the volume of Human TruStain FcX™ for 1x107 cells is 5 µL, add 50 µL for 1 x 108 cells. When working with less than 107 cells, use indicated volumes for 107 cells.
- Add 10 µL of the pre-diluted Biotin-Antibody Cocktail. Mix well and incubate on ice for 15 minutes. Scale up the volume accordingly if separating more cells. For example, add 100 µL of Antibody Cocktail for separating 1 x 108 cells in 1 ml of MojoSort™ Buffer. When working with less than 107 cells, use indicated volumes for 107 cells. Optional: Take an aliquot before adding the cocktail to monitor purity and yield.
- Resuspend the beads by vortexing, maximum speed, 5 touches. Add 10 µL of pre-diluted Streptavidin Nanobeads. Mix well and incubate on ice for 15 minutes. Scale up the volume accordingly if separating more cells. For example, add 100 µL of Nanobeads for separating 1 x 108 cells in 1 ml of MojoSort™ Buffer. When working with less than 107 cells, use indicated volumes for 107 cells.
- Wash the cells by adding MojoSort™ Buffer up to 4 mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard supernatant.
- Resuspend cells in the appropriate amount of MojoSort™ Buffer and proceed to separation. At least 500 µL is needed for column separation.
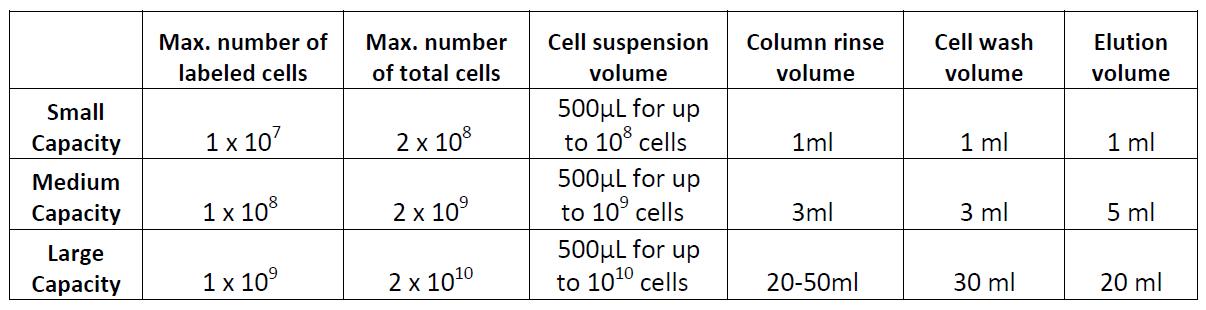
Note: There are several types of commercially available columns, depending on your application. Choose the one that fits best your experiment:
Columns:
Example of magnetic separation with medium capacity columns:
- Place the column in a magnetic separator that fits the column.
- Rinse the column with 3 mL of cell separation buffer.
- Add the labeled cell suspension in at least 500 µL of buffer to the column through a 30 µm filter and collect the fraction containing the unlabeled cells. These are the cells of interest; do not discard.
- Wash the cells in the column 2 times with 3 mL of buffer and collect the fraction containing the untouched cells. Combine with the collected fraction from step 3.
- If desired, the labeled cells can be collected by taking away the column from the magnet and place it on a tube. Then add 5 mL of buffer and flush out the magnetically labeled fraction with a plunger or supplied device. The labeled cells may be useful as staining controls, to monitor purity/yield, or other purposes.
Data
Flow cytometry. High purity and yield. “After Isolation” plot shows purified population of interest using pre-diluted MojoSort™ reagents in separation columns
. 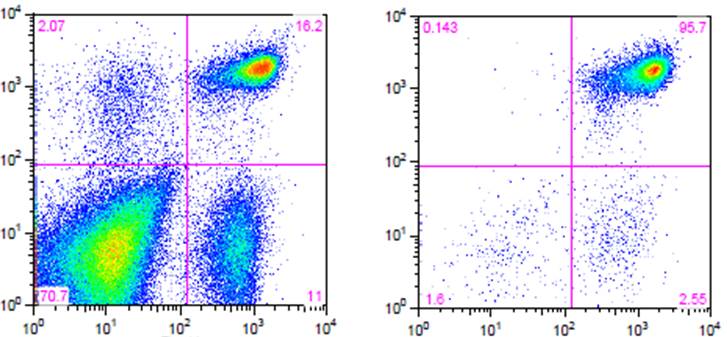
| Kit | Purity | Yield |
|---|---|---|
| Mouse CD4 T Cell Isolation Kit | 95.7% | 85% |
Electron Microscopy. CD4+ T cells Isolated with MojoSort™ CD4 T Cell Isolation Kit using columns do not display particles in the cell surface. Image is representative of 36 different cells.
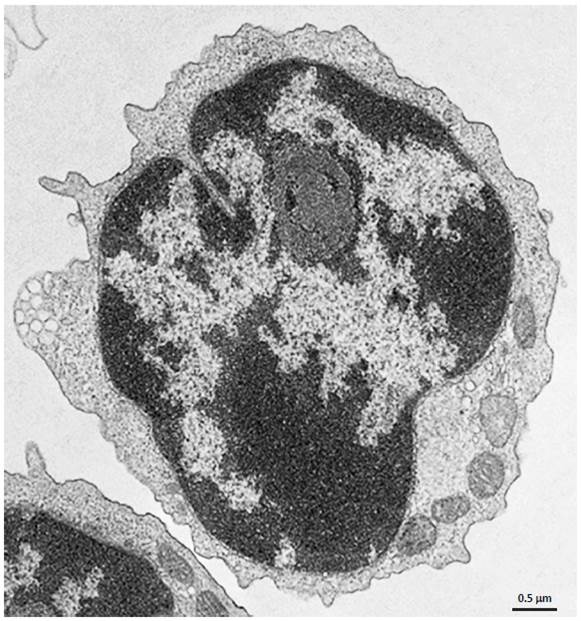
CD4+ T cells isolated with MojoSort™ CD4 T Cell Isolation Kit using separation columns.
 Login / Register
Login / Register 







Follow Us