Optimized Reagents for Alzheimer’s Disease Research
November is Alzheimer’s disease (AD) awareness month, and we’re highlighting the resources our scientists have developed to advance research, including high-quality and specific reagents for the detection of key target proteins involved in the pathogenesis of AD.
Gain a better understanding of the mechanisms and origins of neurodegenerative disorders with our resources for neuroscience research. Our protein- and modification-specific antibodies, recombinant proteins, immunoassays, and reagents are optimized to study protein aggregation and cellular processes involved in neurodegeneration.
To learn more about the known associated proteins that contribute to AD, and to view a comprehensive list of available reagents, visit our Neurodegeneration page.
Neurodegenerative disorders result from the loss of neurons in the nervous system, which can have debilitating effects like dementia, memory loss, and ataxia. Therapies and treatments for these diseases are limited, raising the need to advance research into the causes of these disorders. AD is a progressive and terminal neurodegenerative disorder with limited treatment options, and there is currently no cure. On average, people live three to eleven years once diagnosed, but some may live for 20 years or more depending on the severity of impairment1.
Various inflammatory processes and cytokines are thought to play a role in the pathology of AD. Genetic mutations have also been observed in 1-5% of cases2. Some of these mutations are in the genes encoding amyloid precursor protein (APP). The physiological function of APP is not fully understood. APP is cleaved within the transmembrane domain by γ-secretase to produce amyloid-beta (Aβ). The oligomerization of the Aβ peptides is the primary component of senile plaques found in the brains of AD patients. These plaques are believed to cause cellular toxicity by disrupting the cell's calcium ion homeostasis, inhibiting utilization of glucose by neurons, inducing inflammation and oxidative stress, and ultimately, inducing programmed cell death3.

IHC staining of Spark YG™ 570 anti-β-Amyloid, 17-24 antibody (clone 4G8, red) and DAPI (blue) on formalin-fixed paraffin-embedded Alzheimer's disease brain tissue. Scale bar: 50 µm
Another predominant contributor to AD is Tau hyperphosphorylation. Tau is a microtubule-associated protein (MAP) that stimulates tubulin assembly into axonal microtubules in the brain. Hyperphosphorylated Tau are also large components of neurofibrillary tangles in AD brains.
Phosphorylation and dephosphorylation are part of the normal processing that Tau undergoes in the healthy neuron as a consequence of dynamic regulation of Tau kinases and phosphatases. However, the phosphorylation level of Tau isolated from autopsied AD brains is 3- to 4-fold higher than non-diseased brains4. Despite extensive studies, the causes leading to abnormal hyperphosphorylation of Tau are still largely unknown. Our ELISA MAX™ Deluxe Set Human Total Tau helps researchers detect all six Tau isoforms (2N4R, 1N4R, 0N4R, 2N3R, 1N3R, and 0N3R) of Tau in biological sample types, including cerebrospinal fluid (CSF), plasma, and cell culture supernatant.
In addition, our scientists are developing products and immunoassays for AD research and spatial biology, including antibody conjugates and chemical probes for well-established applications, such as ICC/IHC, or increasingly advanced applications like highly multiplexed imaging of 65+ fluorophore targets with IBEX.
We provide resources and reagents for advancing research in neurodegenerative disorders. The accumulation of amyloid β peptide (Aβ (1-42)) in extracellular plaques is one of the hallmarks of AD5. Aβ (1-40) is also an important biomarker for AD due to the proposed Aβ42/Aβ40 ratio in CSF as an important biomarker of the severity of disease. A decrease in Aβ40 CSF levels has also been implicated in other conditions like frontotemporal dementia. Our LEGEND MAX™ Human Amyloid β 1-42 and LEGEND MAX™ Amyloid β 1-40 ELISA kits are designed for human amyloid protein fragment quantification in several types of human samples, including serum, plasma, and CSF.
Featured LEGENDplex™ Pre-defined Panels
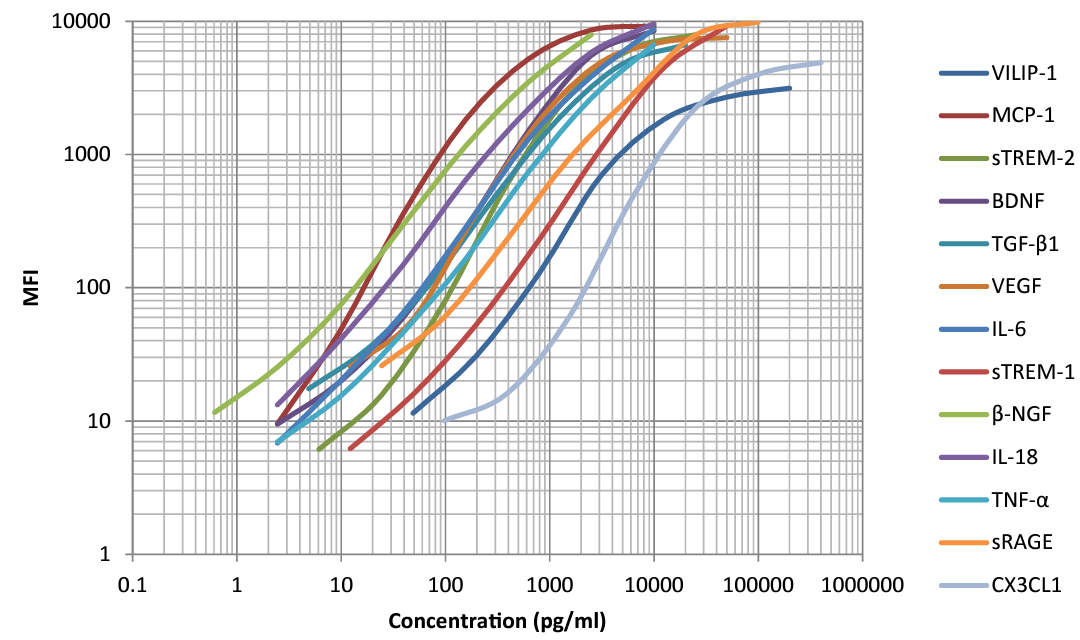
This standard curve was generated using the LEGENDplex™ Human Neuroinflammation Panel 1. The standard curve helps determine the concentration of the vital analytes shown above which can play a role in AD outcome.
Our LEGENDplex™ multiplex panels can simultaneously detect several neuroscience-related biomarkers with fluorescence-encoded beads that can be read on common flow cytometers. The Human Neuroinflammation Panel 1 simultaneously quantifies 13 key targets involved in neuroinflammation such as VILIP-1, CCL2 (MCP-1), sTREM-2, BDNF, Free Active TGF-β1, VEGF, IL-6, sTREM-1, β-NGF, IL-18, TNF-α, sRAGE, and CX3CL1 (Fractalkine). Our LEGENDplex pre-defined panels are designed to facilitate your research across different research areas. These pre-optimized panels can also provide higher detection sensitivity with broader dynamic range than traditional ELISA methods.
For quantifying known biomarkers in neurodegenerative disorders, our LEGENDplex Human Neurodegenerative Disease Biomarker Panel 1 is designed to measure five analytes simultaneously (α-Synuclein, Tau, Aβ40, Aβ42, and Neurofilament-L). For analytes that cross multiple panels or for panels requiring custom analytes, talk with our dedicated Custom Solutions Team so we can build you the ideal research tools.
More Reagents for Breakthroughs
We are dedicated to empowering you with the right applications and tools to conduct your research. For the analysis of other cell types in the brain, we provide reagents for isolation, such as the MojoSort™ Mouse P2RY12 Selection Kits and a diverse array of directly conjugated anti-P2RY12 antibodies.

IHC staining of purified anti-P2RY12 antibody (clone S16007D, magenta) on FFPE mouse brain tissue. Nuclei were counterstained with DAPI. Scale bar: 50 µm

Glial cell-derived neurotrophic factor (GDNF) is a member of the TGF-β superfamily that signals through GDNF family receptor α-1 (GFRα1) to promote the development, differentiation, and survival of dopaminergic neurons. Our GMP Ultra-LEAF™ Purified anti-GDNF antibody (clone A16099G, Cat. No. 670905) inhibits the binding of recombinant human GFRα1 to immobilized recombinant human GDNF (Cat. No. 760402) in a dose-dependent manner. This antibody neutralizes the binding of GFRα1 protein to GDNF, allowing researchers to examine neuronal growth and progression in disease and treatment models.
As more markers and methods are discovered, we remain determined to create the reagents and resources needed to advance all aspects of your Alzheimer’s disease, neuroscience and neurodegeneration research, including useful protocols for whole mouse brain processing, astrocyte isolation and culturing, and our complimentary resource guide. Make a difference in your neuroscience research by trusting the high-quality and validated reagents created by our scientists.
References
- Mayo Clinic Staff. "Alzheimer's stages: How the disease progresses." June 7, 2023. https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/in-depth/alzheimers-stages/art-20048448
- Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012. 1;2(10):a006296. doi: 10.1101/cshperspect.a006296. PMID: 23028126; PMCID: PMC3475404.
- Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, Villemagne VL, Aisen P, Vendruscolo M, Iwatsubo T, Masters CL, Cho M, Lannfelt L, Cummings JL, Vergallo A. The Amyloid-β Pathway in Alzheimer's Disease. Mol Psychiatry. 2021. 26(10):5481-5503. doi: 10.1038/s41380-021-01249-0. PMID: 34456336; PMCID: PMC8758495.
- Wegmann S, Biernat J, Mandelkow E. A current view on Tau protein phosphorylation in Alzheimer's disease. Curr Opin Neurobiol. 2021. 69:131-138. doi: 10.1016/j.conb.2021.03.003. PMID: 33892381.
- Gouras GK, Olsson TT, Hansson O. β-Amyloid peptides and amyloid plaques in Alzheimer's disease. Neurotherapeutics. 2015. 12(1):3-11. doi: 10.1007/s13311-014-0313-y. PMID: 25371168; PMCID: PMC4322079.
 Login / Register
Login / Register 






Follow Us