Obese Flies and Other Diabesity Tools
Our knowledge of cellular metabolism and the regulation of energy homeostasis has come a long way, but is still incomplete. Ongoing research of the molecular pathophysiology of metabolic diseases, such as diabetes and obesity, will help fill in some of the gaps. This research is also critical to help us move toward treatment of these pathologies.
Research using animal models like the fruit fly, Drosophila melanogaster, certainly helps as it turns out that the molecular mechanisms underlying energy homeostasis are largely conserved between humans and flies. Like humans, fruit flies love sugar and fat and can become obese on high-sugar or high-fat diets[1]. The resulting obese flies develop phenotypes akin to cardiomyopathy and diabetes. We need tools, such as animal models and our LEGENDplex™ panels and ELISA kits, to help decipher the complexity of metabolic disorders.
Worldwide, the increase in the percentage of overweight and obese people over recent decades is now considered an epidemic (Fig 1). The nutritional status of individuals is classified by BMI (body mass index equals a person’s weight in kg divided by person’s height in meters, squared), with the World Health Organization (WHO) defining overweight as BMI of 25-29.9 kg/m2 and obesity as BMI ≥ 30 kg/m2 [2]. However, in Asian populations, a BMI ≥ 27.5 kg/m2 is the recommended cutoff for obesity classification due to increased risk of certain co-morbidities[3].
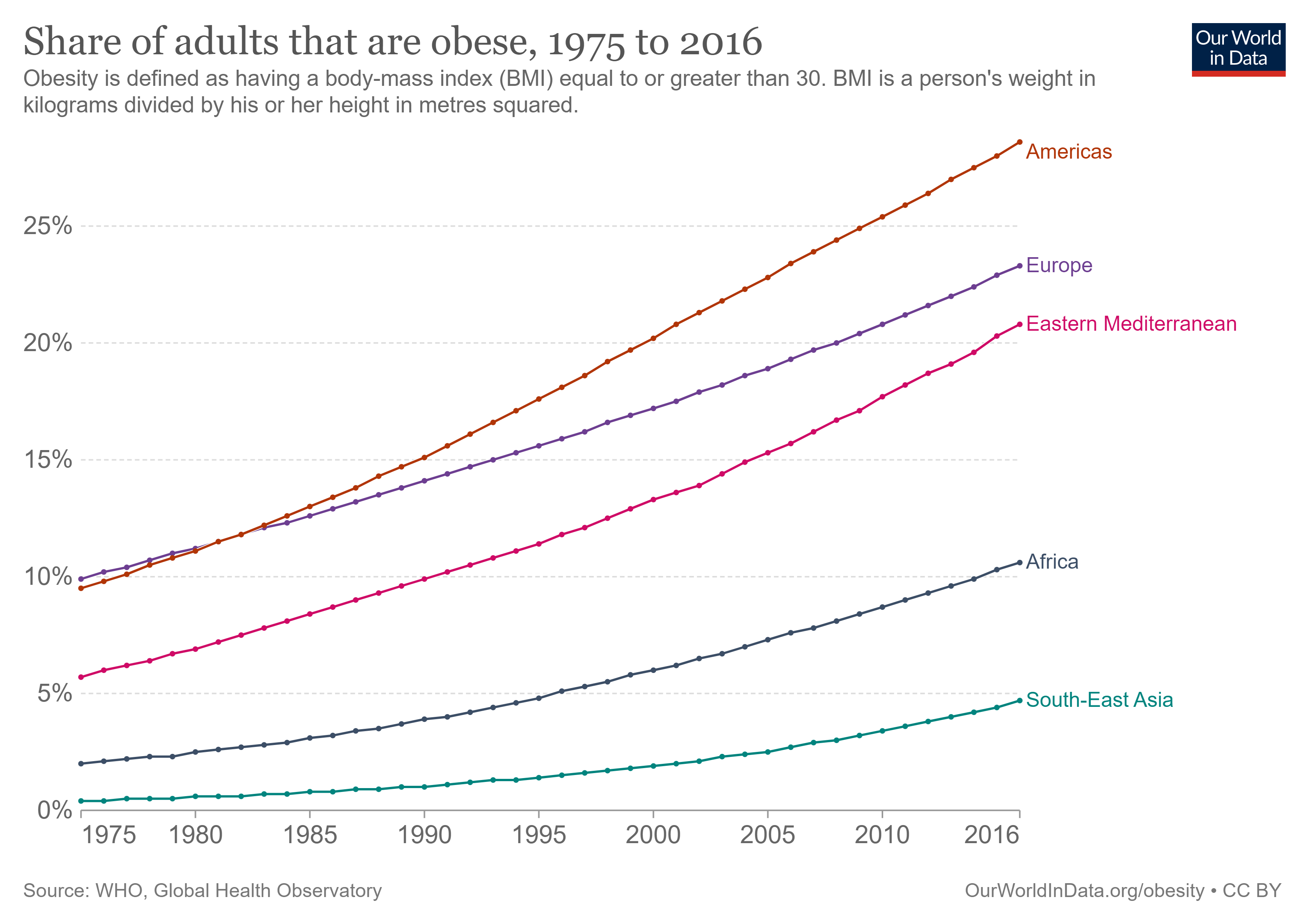
Figure 1. Number of clinically obese adults from 1975 to 2016 in selected global regions[4].
Likewise, deaths attributed to obesity has increased globally on average from 4.5% in 1990 to 8% in 2019[4] (Fig 2).

Figure 2. Percentage of deaths attributed to obesity from 1990 to 2019 in selected global regions[4].
Obesity occurs when the human body experiences an excess intake of food energy over the output for that energy. The management of a chronic excess of caloric energy falls the hardest on metabolic organs (adipose tissue, pancreas, and the liver) and can lead to metabolic pathologies/co-morbidities such as type 2 diabetes (T2D), hyperlipidemia/dyslipidemia, cardiovascular risk, nonalcoholic fatty liver disease (NAFLD) and some cancers[5].
In this blog, we focus on the metabolic disease of T2D, one of the world’s leading causes of death that is also associated with obesity. In fact, the term diabesity is used to describe the occurrence of T2D in relation to obesity[6]. T2D is a metabolic disease brought about by impaired insulin production by pancreatic β-cells as well as the development of insulin resistance in key tissues such as adipose, muscles, and liver. In turn, these insulin-related alterations lead to hyperglycemia. If left untreated, this can then cause blindness, renal failure, cardiovascular disease, and lower-limb amputations.
We now know that adipose, long thought to just store fat, is actually a complex, endocrine organ that plays an important role in whole body homeostasis. It secretes a number of bioactive compounds, like hormones termed adipokines and cytokines. In obesity, adipose tissue undergoes hypertrophy and the tissue microenvironment takes on a proinflammatory phenotype. As such, obese adipose tissue tends to be associated far more with macrophages of the M1 phenotype that secrete proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-18, and CXCL5, among others[7] (Fig 3). Adipose also plays an important role mediating effects of insulin. For example, obese adipocytes secrete adipokines implicated in insulin resistance, such as resistin, RBP-4, MCP-1, progranulin, chemerin, and ANGPTL2. This is noted to occur with a concomitant decrease in insulin-sensitizing adipokines, such as adiponectin, leptin, apelin, omentin, secreted frizzled-protein 5, and anti-inflammatory cytokines IL-10 and IL-22[7,8] (Fig 3).
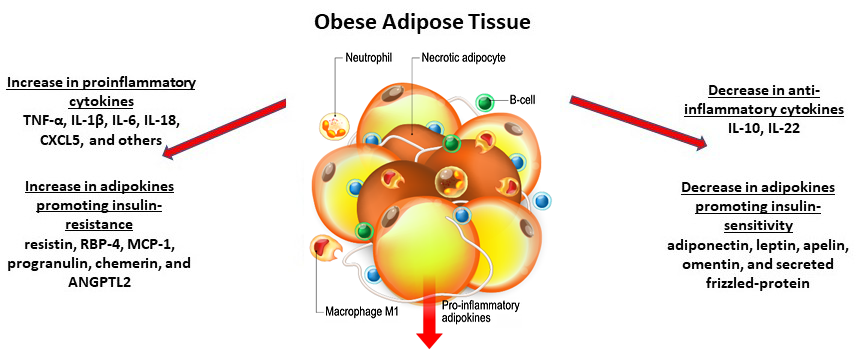
Figure 3. Obese adipose tissue and associated macrophages and cytokines.
Clearly, cytokines and adipokines are key biomarkers and regulators in obesity and associated pathologies like T2D. Quantitative measurement of these compounds and changes to their expression level are critical to better understand their roles within normal and pathological conditions. It is with such data that we can link cytokines and adipokines to obesity-associated pathologies and their underlying mechanisms, and, importantly, gain insight into the efficacy of therapeutics.
To get precise measurements, the enzyme-linked immunosorbent assay (ELISA) is a commonly used method to quantify individual specific cytokines in samples. On the other hand, we offer LEGENDplex, multiplex flow-based assays in which reactions take place on beads, with each bead conjugated to a specific antibody on its surface that serves to capture a target analyte. Each population of beads can be distinguished by size and internal fluorescence, allowing for the simultaneous analysis of multiple target analytes in a sample. With the addition of a biotinylated detection antibody, this process bears similarities to the classical sandwich ELISA. But, the end readout is made after adding Streptavidin-PE reagent, using a flow cytometer for data acquisition.
To understand the network of cytokines in T2D, we developed:
- LEGENDplex Human Diabesity Panel (11-plex) to help researchers simultaneously quantify proinflammatory cytokines implicated in diabesity, such as TNF-α, IL-1β, and IL-6.
- LEGENDplex Human Adipokine Panel (13-plex) to quantify adipokines and cytokines that induce insulin-sensitization or resistance, including adiponectin, adipsin, leptin, resistin, RBP-4, MCP-1, and IL-10.
- LEGENDplex Human Metabolic Panel 1 that specifically targets the four adipokines adiponectin, adipsin, leptin, and resistin.
Now that it is well understood that adipose plays a role in metabolic homeostasis, it is not surprising that adipose is a target for therapeutics. One potential therapeutic is Fibroblast Growth Factor 21 (FGF-21), a peptide hormone produced by the liver that acts on adipocytes. In adipose, FGF-21 seems to have anti-diabetic properties as it stimulates glucose uptake in an insulin-independent manner. Use our ELISA MAX™ Deluxe Set Human Fibroblast Growth Factor 21 to clarify the role of FGF-21 and its potential as a therapeutic agent for T2D and other metabolic disorders[9].
Beyond its role in regulating food intake and body weight, leptin has emerged as an adjunct to insulin as a powerful modulator of glucose, lipid, and protein metabolism[10]. However, more research is needed to fully decipher the role of dysregulated leptin in diabesity and its therapeutic potential. Toward this goal, we offer the ELISA MAX Deluxe Set Human Leptin to quantify natural and recombinant leptin in diverse biological fluids. Studies of antidiabetic agents that modulate serum leptin levels in physiological and pathophysiological conditions are underway. For example, dipeptidyl peptidase-4 (DPP-4) inhibitors are emerging as agents for reducing blood glucose levels and improving insulin resistance[11]. Use our Human DPP-4 ELISAs to accurately quantify this multi-functional protein (also known as T-cell differentiation antigen CD26 in immune cells) and its potential link between obesity and the pathogenesis of T2D and metabolic disease.
Obesity and co-morbidities such as T2D are escalating worldwide as public health problems. Researchers are making great strides at understanding how genetics and environmental factors interact to define the etiology of these conditions, but work remains. Learn more about our ELISA kits and multiplex assays, which allow you to discover and characterize molecular responses associated with pathologies such as diabesity. Using tools such as animal models and our immunoassays, researchers can discover and start mapping out the multiple molecular factors underlying diabesity. Together, we can make progress toward treating metabolic pathologies associated with obesity.
References
- Agrawal, Neha et al. “Predicting novel candidate human obesity genes and their site of action by systematic functional screening in Drosophila.” PLoS biology vol. 19, 11 e3001255. 8 Nov. 2021, doi:10.1371/journal.pbio.3001255. PubMed
- Obesity and Overweight. 9 June 2021, www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- Purnell, Jonathan. “Definitions, Classification, and Epidemiology of Obesity.” Edited by KR Feingold et al., Endotext [Internet], 2018, https://www.ncbi.nlm.nih.gov/books/NBK279167/.
- Ritchie, Hannah, and Max Roser. “Obesity.” Our World in Data, 11 Aug. 2017, https://ourworldindata.org/obesity.
- Suárez-Cuenca, Juan Antonio et al. “Enlarged adipocytes from subcutaneous vs. visceral adipose tissue differentially contribute to metabolic dysfunction and atherogenic risk of patients with obesity.” Scientific reports vol. 11,1 1831. 19 Jan. 2021, doi:10.1038/s41598-021-81289-2. PubMed
- Reed, Josh et al. “A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives.” Diabetes, metabolic syndrome and obesity : targets and therapy vol. 14 3567-3602. 10 Aug. 2021, doi:10.2147/DMSO.S319895 d J., et al. 2021. Diabetes Metab Syndr Obes. 14:3567. PubMed
- Banerjee, Amrita, and Jagdish Singh. “Remodeling adipose tissue inflammasome for type 2 diabetes mellitus treatment: Current perspective and translational strategies.” Bioengineering & translational medicine vol. 5,2 e10150. 13 Dec. 2019, doi:10.1002/btm2.10150. PubMed
- Recinella, Lucia et al. “Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases.” Frontiers in physiology vol. 11 578966. 30 Oct. 2020, doi:10.3389/fphys.2020.578966. PubMed
- Queen, Nicholas J et al. “Visceral adipose tissue-directed FGF21 gene therapy improves metabolic and immune health in BTBR mice.” Molecular therapy. Methods & clinical development vol. 20 409-422. 25 Dec. 2020, doi:10.1016/j.omtm.2020.12.011. PubMed
- Pereira, Sandra et al. “Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism.” Endocrine reviews vol. 42,1 (2021): 1-28. doi:10.1210/endrev/bnaa027. PubMed
- Wei, Xin et al. “Association between dipeptidyl peptidase-4 inhibitors use and leptin in type 2 diabetes mellitus.” Diabetology & metabolic syndrome vol. 13,1 88. 26 Aug. 2021, doi:10.1186/s13098-021-00703-x. PubMed
 Login / Register
Login / Register 






Follow Us