Biotinylated Recombinant Proteins
Biofunctional biotinylated recombinant proteins are excellent tools for molecular research and bioassays. The core concepts of biotinylation and recombinant proteins were created decades ago, and the combination of this technology allows us to offer a wide selection of reagents for various applications including IHC, ELISAs and flow cytometry.
Explore our full list of biotinylated recombinant proteins.
Production of recombinant proteins is essential for the development of novel drugs, as well as the discovery of potential drug targets. Recombinant proteins are specifically designed by encoding a gene of interest followed by cloning and expressing it in a system for translation of the desired protein. By controlling the sequence of the gene of interest, recombinant proteins can be designed with precision and modifications, such as truncated or tagged versions.
The idea of recombinant DNA and the method of producing it were developed by graduate student Peter Lobban of Stanford University. Lobban, along with his professor Dale Kaiser, published their hypothesis titled Enzymatic end-to end joining of DNA molecules (1973) and explained the method of separating and amplifying genes and inserting them into a new host cell1. In 1974, Stanford University applied for a patent with Stanley Cohen and Herbert Boyer as inventors3, and it was later awarded in 1980. In the following years, important advancements in the technology arose, such as the creation of the biosynthetic human insulin by Herbert Boyer and colleagues.
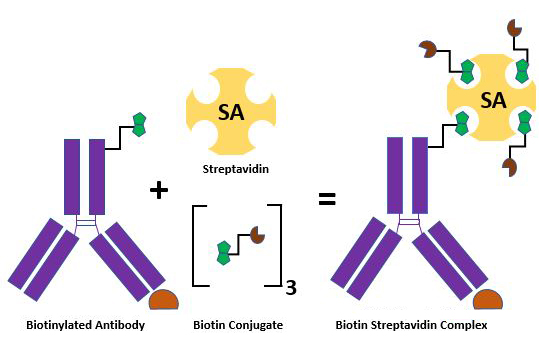
The term biotinylation refers to the process of adding a biotin molecule to a nucleic acid or protein. Biotinylation is widely used for labeling and purification of proteins. The process consists of covalently attaching biotin to a protein of interest and is designed to leverage biotin’s strong affinity with Streptavidin and Avidin molecules. For example, the binding of a biotinylated protein to a receptor could easily be measured by the addition of a streptavidin reagent conjugated to horseradish peroxidase (HRP). This process is relatively quick, specific, and does not significantly affect biological activity.
Schematic showing a biotinylated antibody and the formation of a complex with Streptavidin and biotin conjugates.
This method is used extensively in molecular biology and biotechnology due to the streptavidin-biotin complex's resistance to denaturants, detergents, organic solvents, proteolytic enzymes, and extremes of temperature and pH. The interaction between streptavidin and biotin is considered to be one of the strongest non-covalent interactions between a protein and ligand, making it incredibly useful for targeting proteins of interest2.
Biotinylated proteins can be used for researching low-abundance proteins or protein-protein interactions. The process of biotin labeling is frequently used as an alternative to radiation methods of protein labeling.
Our biotinylated recombinant proteins are useful for high throughput screening of potential protein-protein interaction blocking molecules or discovery of target cells in tissues or blood. Some additional potential uses include isolation of target proteins or cells with magnetic beads and plate-based immunoassays.
Our team recently developed biotinylated recombinant SARS-CoV-2 S Protein S1 and S protein RBD for the study of the viral pathways involved in COVID-19 infection and pathology. These reagents can be useful for isolation of target proteins or screening of potential protein-protein interactions. Our featured data shows biotinylated recombinant SARS-CoV-2 S Protein S1 binding to a recombinant ACE2-Fc chimera. ACE2 is one of the more well-known binding receptors for SARS-CoV-2’s spike proteins. Learn more about SARS-CoV-2 proteins with our helpful webpage.
Monomeric streptavidin (blue) with bound biotin4.
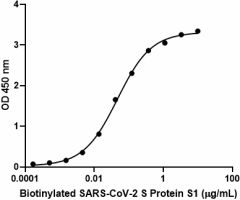
When recombinant ACE2-Fc chimera is immobilized at 1 µg/mL, biotinylated recombinant SARS-CoV-2 S Protein S1 binds in a dose-dependent manner. The EC50 range for this effect is 20–100 ng/mL. HRP Avidin was used to detect binding.
Additionally, our TotalSeq™ oligonucleotide and fluorophore conjugated streptavidin allows for the multimerization of SARS-CoV-2 antigens, provides a unique antigen barcode, and enables fluorescent-based cell sorting prior to sequencing. View our TotalSeq™-C Streptavidin Reagents.
Biotinylated recombinant proteins are widely used in molecular research and are important for multiple aspects of drug development. The interaction between streptavidin and biotin is one of the strongest non-covalent interactions between a protein and ligand, making it incredibly useful for biofunctional research, such as targeting proteins of interest or high throughput screening of protein-protein interactions. Our biotinylated recombinant proteins are manufactured by experts and quality tested at the highest possible standard for bioactivity and include cytokines, growth factors, chemokines, and enzymes. If you would like us to produce a custom recombinant protein, contact our custom solutions team.
References
- Lobban PE and Kaiser AD. 1973. Enzymatic end-to end joining of DNA molecules. J Mol Biol. 78(3):453-71. DOI: 10.1016/0022-2836(73)90468-3. PMID: 4754844.
- Bratthauer G. 2009. The Avidin-Biotin Complex (ABC) Method and Other Avidin-Biotin Binding Methods. Immunocytochemical Methods and Protocols. Humana Press, 257-270. DOI: 10.1007/978-1-59745-324-0_26
- Cohen SN, et al. 1973. Construction of biologically functional bacterial plasmids in vitro. Proceedings of the National Academy of Sciences of the United States of America. 70(11), 3240-3244. DOI: 10.1073/pnas.70.11.3240
- Weber P, et al. 1989. Structural origins of high-affinity biotin binding to streptavidin. Science 243: 85-88. DOI: 10.1126/science.2911722
 Login / Register
Login / Register 






Follow Us