- Regulatory Status
- RUO
- Other Names
- Scatter Factor (SF), Hepatopoietin (HPTA), Lung fibroblast-derived mitogen
- Ave. Rating
- Submit a Review
- Product Citations
- publications
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 596401 | 2 µg | 94 CHF | ||||
| 596402 | 10 µg | 218 CHF | ||||
Hepatocyte growth factor (HGF )was initially purified from plasma of a patient with fulminant hepatic failure. HGF is a dimeric molecule composed of the α-subunit and β-subunit, linked by a disulfide bond. The two chains are derived from a precursor encoded by the same open reading frame. The C-terminus of the α-subunit is followed directly by the N-terminus of the β-subunit. The pro-HGF possesses 728 amino acids and the subunits are formed by proteolytic cleavage. Proteolytic activation of the precursor form in the extracellular milieu is performed by HGF activator (HGFA). HGFA is a factor XII-like serine proteinase that is not inhibited by serum proteinase inhibitors. Two inhibitors have been described for HGFA, HGFA inhibitor type 1 and type 2 (HAI-1 and -2). Both inhibitors have two well-defined Kunitz inhibitor domains and a presumed transmembrane domain. HGF protects the epithelium, neurons and cardiomyocytes during organ diseases; this protective effect is mediated through anti-apoptotic signals via inhibition of caspase-3 activity or induction of anti-apoptotic molecules, such as Bcl-xL. In addition, HGF prohibits Fas-mediated apoptosis signals via sequestration of Fas and Met receptor on cell surfaces. Also, HGF elicits the regression of fibrosis in numerous organs, such as scleroderma, cardiomyopathy, vocal scarring, and peritoneal fibrosis.
Product DetailsProduct Details
- Source
- Human HGF, amino acids (Gln32- Arg494 and Val495 - Ser728) (Accession# NM_000601), was expressed in insect cells.
- Molecular Mass
- The alpha and beta chains contains 463 and 234 amino acids and have a predicted molecular mass of approximately 53.6 and 26 kD respectively. The predicted N-terminal amino acids are Gln and Val.
- Purity
- >98%, as determined by Coomassie stained SDS-PAGE and HPLC analysis.
- Formulation
- Lyophilized
- Endotoxin Level
- Less than 0.1 ng per µg of protein.
- Storage & Handling
- Unopened vial can be stored at -20°C or -70°C. For maximum results, quick spin vial prior to opening. Reconstitute in water to a concentration of 0.5 mg/ml. Do not vortex. Note: Allow the reconstituted vial to sit at room temperature for 1 hour before use. It is recommended to further dilute in a buffer containing a carrier protein such as 0.1% BSA and store working aliquots at -20°C to -80°C. Avoid repeated freeze/thaw cycles.
- Activity
- ED50 = 20 - 40 ng/ml, corresponding to a specific activity of 2.5 - 5.0 x 104 units/mg, as determined by induction of 4MBr-5 cell proliferation.
- Application
-
Bioassay
-
Application References
(PubMed link indicates BioLegend citation) -
- Nakamura T, et al. 2011. J. Gastroenterol. Hepatol. Suppl. 1:188.
Antigen Details
- Structure
- Heterodimer (alpha and beta chains)
- Distribution
- Mesenchymal cells, liver sinusoidal endothelial, macrophages.
- Function
- Stimulates epithelial cell proliferation, motility, morphogenesis and angiogenesis. Hypoxia and hyperglycemia suppress HGF production. Also, TGF-β and angiotensin-II depress HGF production in vitro.
- Interaction
- Hepathocytes, epithelial cells.
- Ligand/Receptor
- MET receptor
- Cell Type
- Embryonic Stem Cells
- Biology Area
- Angiogenesis, Cell Biology, Stem Cells
- Molecular Family
- Growth Factors, Cytokines/Chemokines
- Antigen References
-
1. Kataoka H, et al. 2000. J. Biol. Chem. 275:40453.
2. Wang X, et al. 2002. Mol. Cell 9:411.
3. Wu MH, et al. 2004. Gene Ther. 11:170.
4. Mizuno S, et al. 2008. Front. Biosci. 13:7072.
5. D'Angelo F, et al. 2013. Clin. Exp. Immunol. 174:60. - Gene ID
- 3082 View all products for this Gene ID
- UniProt
- View information about HGF on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
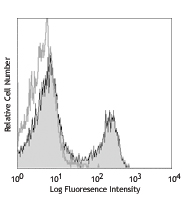
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login / Register
Login / Register 






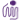




Follow Us