- Regulatory Status
- RUO
- Other Names
- FUR, PACE, PCSK3, SPC1
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

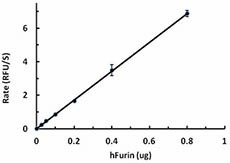
The activity of human Furin was measured with 50 µM of fluorogenic substrate, pERTKR-AMC , in the presence of 0.025, 0.05, 0.1, 0.2, 0.4, 0.8 ug of hFurin. The specific activity of hFurin is greater than 75 pmole/min/µg.
Furin belongs to the family of highly specific, calcium-dependent proprotein/prohormone convertases (PCs). It is expressed as a 794-amino-acid type-I transmembrane protein that is found in all vertebrates and many invertebrates. Furin and the other PCs contain prodomains that are flanked by the signal peptidase cleavage site on the amino-terminal side and by a conserved set of basic amino acids that comprise the autoproteolytic cleavage site on the carboxy-terminal side. The 83-amino-acid furin prodomain acts as an intramolecular chaperone that guides the folding and activation of the endoprotease. The release of furin's propeptide fragments by autocatalysis unmasks its endoprotease activity and enables it to cleave substrates where it is localized. The consensus site that furin cleaves is positioned after the carboxy-terminal arginine (Arg) residue in the sequence -Arg-X-Lys/Arg-Arg↓- (where X is any amino acid and ↓ identifies the cleavage site). Furin localizes to the Trans-Golgi Network (TGN). From the TGN, furin follows a highly regulated trafficking itinerary through several TGN/endosomal compartments and the cell surface. Some of the unique furin substrates include TGF-β-like precursors such as BMP10 during embryonic development, insulin-like growth factor-1 (IGF-1), membrane-type matrix metalloproteinases, and the iron-regulating protein hepcidin. Infectious agents also utilize furin-like cleavage as a requisite step of their infectious cycle. Numerous bacterial toxins are activated by furin-like cleavage on the cell surface, as occurs for anthrax toxin, or during their endocytic entry, as occurs for Pseudomonas exotoxin. Furin-like cleavage of some viral envelope proteins, including those of avian influenza virus, HIV-1 and measles virus, is required for infectious virion assembly. Furin is upregulated in several cancers, including non-small-cell lung carcinomas, squamous cell carcinomas of the head and neck, and glioblastoma.
Product DetailsProduct Details
- Source
- Human Furin, amino acids Asp108-Glu715 (Accession #BC012181) with a C-terminal TG-8H-GGQ tag was expressed in CHO cells.
- Molecular Mass
- The mature 621 amino acid recombinant protein has a predicted molecular mass of approximately 67.1 kDa. The non-reduced and DTT-reduced proteins migrate at 65 - 75 and 70 - 80 kDa, respectively, by SDS-PAGE.
- Purity
- >90%, as determined by Coomasie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in pH 8.0 buffer containing 10 mM TRIS, 1 mM CaCl2, 100 mM NaCl, and 50% glycerol.
- Endotoxin Level
- Less than 1.0 EU per µg of protein as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored at -20 °C or -70°C for six months. For maximum results, quick spin vial prior to opening. Avoid repeated freeze/thaw cycles.
- Activity
- The activity of human Furin is measured with 50 µM of fluorogenic substrate, pERTKR-AMC, with a specific activity value greater than 75 pmol/ug/min.
- Application
-
Bioassay
- Application Notes
-
Human Furin activity is measured by its ability to cleave a fluorogenic peptide substrate pERTKR-AMC. The increase of the product is monitored by increase in intensity of fluorescence at 460 nm with excitation at 380 nm.
Materials
- Assay Buffer: pH 9.0, 25 mM Tris, 1 mM CaCl2, 0.5% Brij-35
- Recombinant Human Furin
- Furin Substrate: pERTKR-AMC
Activity assay procedures
- Dilute hFurin in the assay buffer at 4 µg/mL.
- Dilute the substrate at 100 µM in Assay Buffer.
- Load into a well plate 50 μL of the 4 ng/μL hFurin and start the reaction by adding 50 μL of 100 μM Substrate. Include a substrate blank containing 50 μL of Assay Buffer and 50 μL of 100 μM Substrate solution.
- Read the product formation by measuring 380/460 nm (Excitation/Emission) in kinetic mode for 5 minutes.
The final hFurin concentration is 2 µg/mL (200 ng) and the substrate is 50 µM.
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com. - Product Citations
-
Antigen Details
- Structure
- Monomer
- Distribution
-
Low expression in US-O2 cell line and moderate expression in U251-MG cell line have been observed.
- Interaction
- TGF-β, MT-MMPs, IGF-1
- Bioactivity
- Proprotein or prohormon convertase
- Molecular Family
- Enzymes and Regulators
- Antigen References
-
1. Dahms S. O. et al. 2016. PNAS 113:11196.
2. Seidah N. G. et al. 2013. J. Biol. Chem. 288(30):21473-81.
3. Day P. M. and Schiller J. T. 2009. Future Microbiol. 4:1255.
4. Thomas G. 2002. Nat. Rev. Mol. Cell. Biol. 3:753.
5. Nakayama K. 1997. Biochem. J. 327( Pt 3):625-35.
6. Matthews D. J. 1994. Protein Sci. 3:1197. - Gene ID
- 5045 View all products for this Gene ID
- UniProt
- View information about Furin on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login / Register
Login / Register 








Follow Us