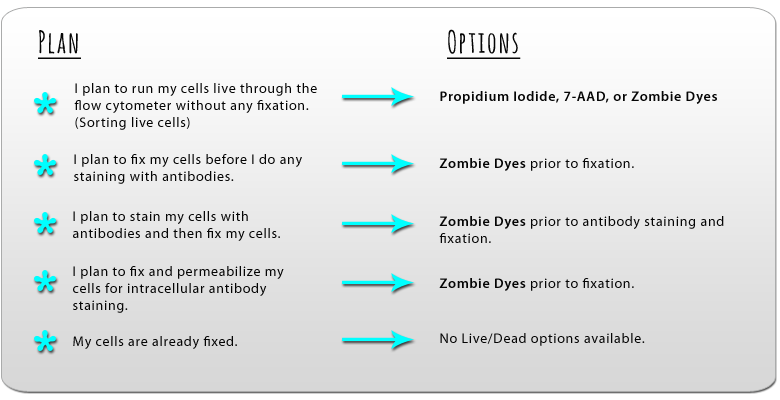
It is critical to understand the degree of cell death in any flow cytometry assay and exclude those cells from the analysis. We provide DNA dyes, including Helix NP™ NIR, DRAQ7™, Propidium Iodide and 7-AAD, that enter and stain dead cells, but are impermeable to live cells for rapid, cost-effective analysis of unfixed cells. In cases where cell fixation is required, we provide fixable Zombie Dyes. Not sure which Zombie is right for you? Try our Zombie Fixable Viability™ Sampler Kit, which contains 100 tests each of the UV, NIR, Violet, Aqua, and Yellow variants.

Zombie Dyes
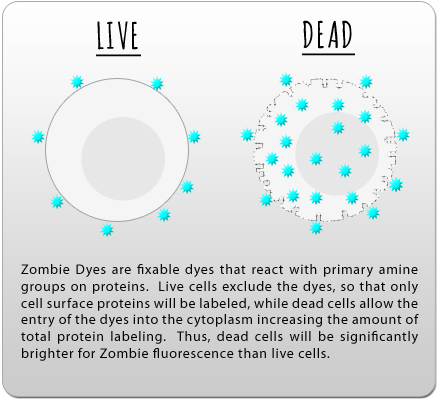
*DRAQ7™ is a trademark of Biostatus Limited.
Helix NP™ NIR, DRAQ7™, PI and 7-AAD
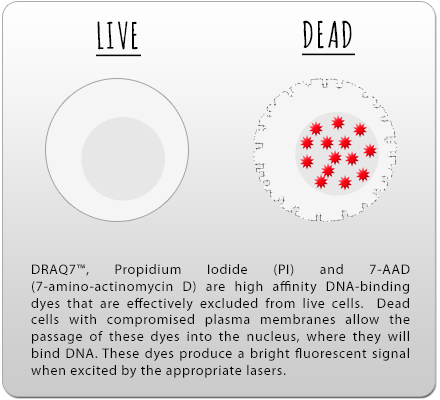
Product Links: Helix NP™ NIR, DRAQ7™, PI, 7-AAD
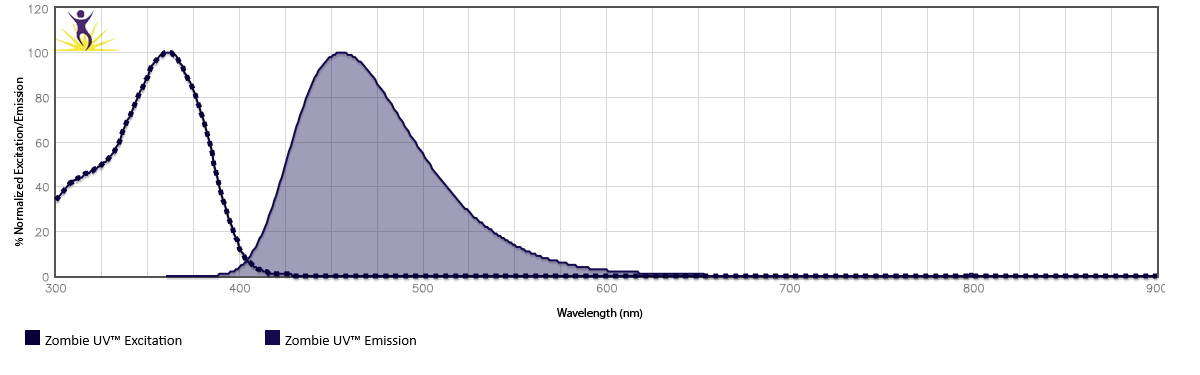
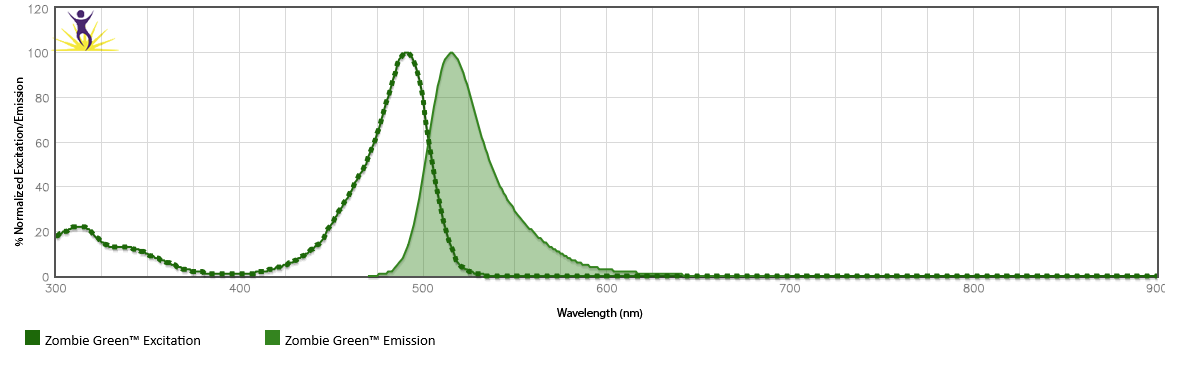
Zombie Dyes by Excitation Laser
Click the plus next to each excitation laser to see the available Zombie Dyes and their corresponding spectra.
Or, examine them on our spectra analyzer tools.
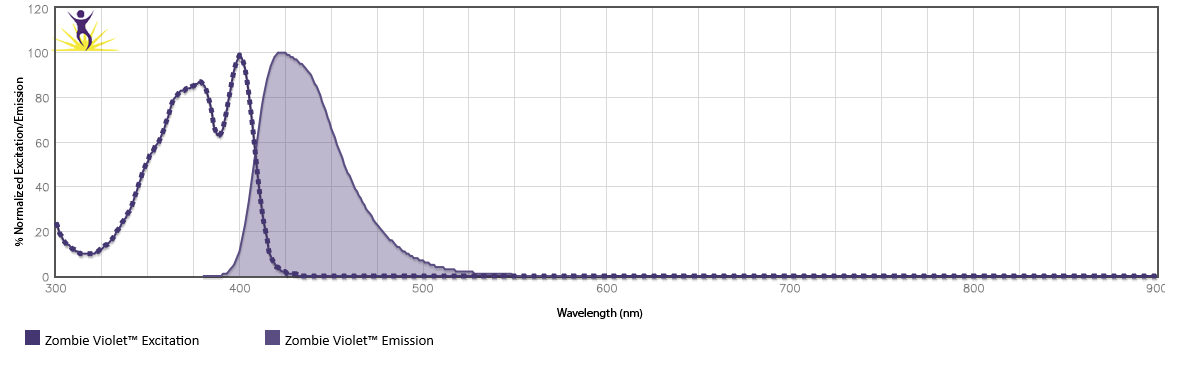
Zombie Violet™ is excited by the violet laser (405 nm) and emits maximally at 423 nm. It has similar emission to Brilliant Violet 421™ and Pacific Blue™.
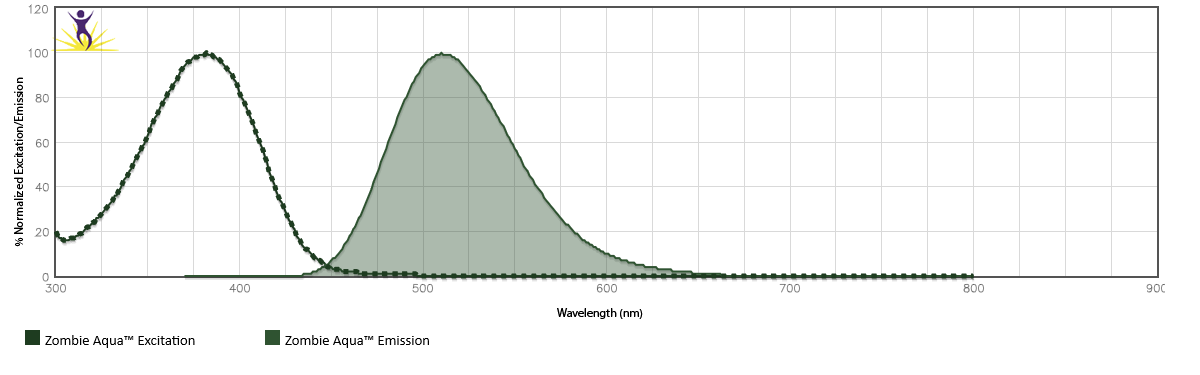
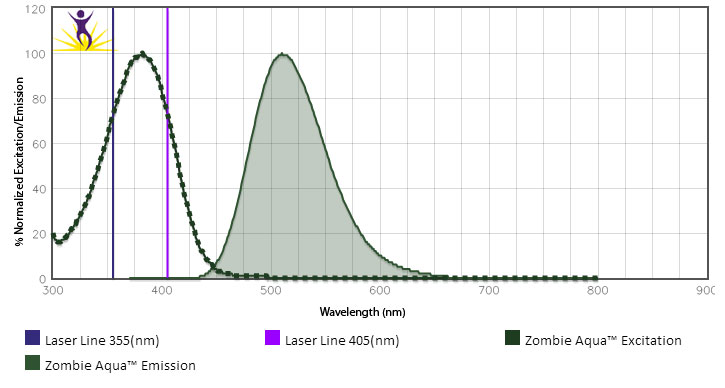
Zombie Aqua™ is excited by the violet laser (405 nm) and the ultraviolet laser (350 nm). It emits maximally at 516 nm.
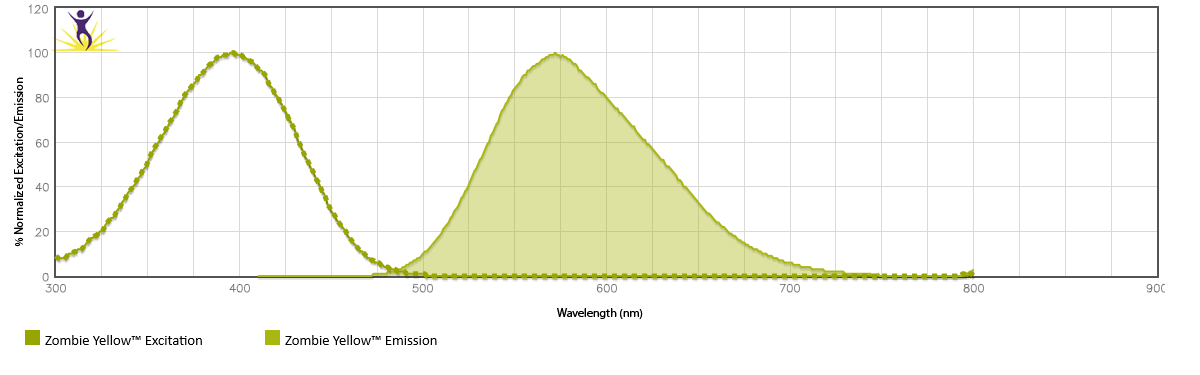
Zombie Yellow™ is excited by the violet laser (405 nm) and partially by the UV laser (350 nm). It emits maximally at 572 nm and has a similar emission profile to Brilliant Violet 570™.
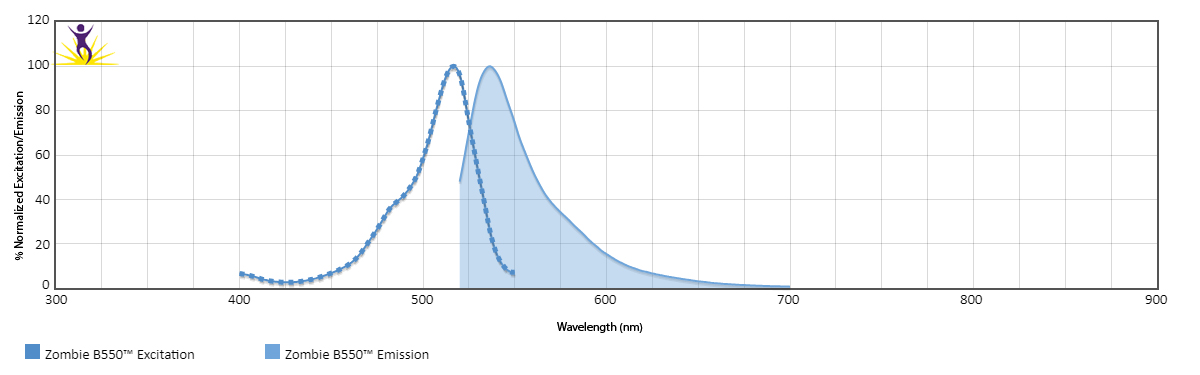
Zombie B550™ is excited by the blue laser (488 nm) and emits maximally at 540 nm. It has similar emission spectra to Spark Blue™ 550.
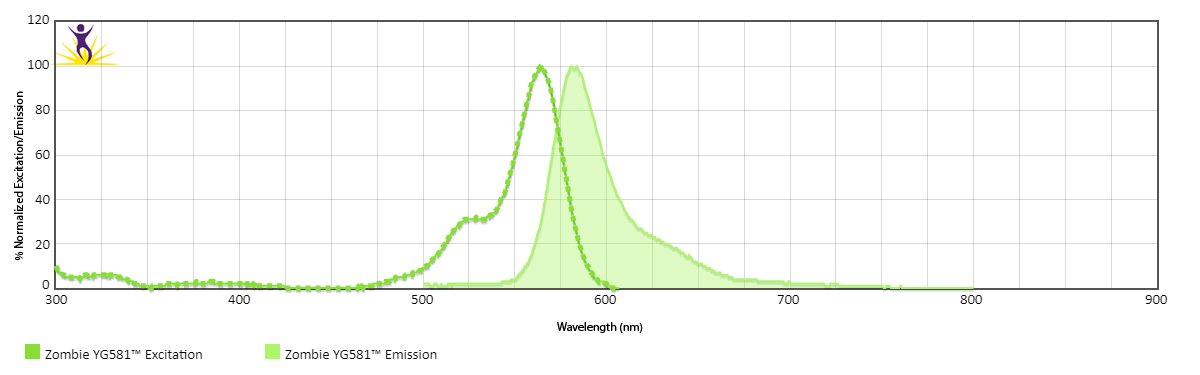
Zombie YG™ 581 is excited by the yellow/green laser (561 nm) and emits maximally at 581 nm. It has similar emission spectra to Spark YG™ 581.
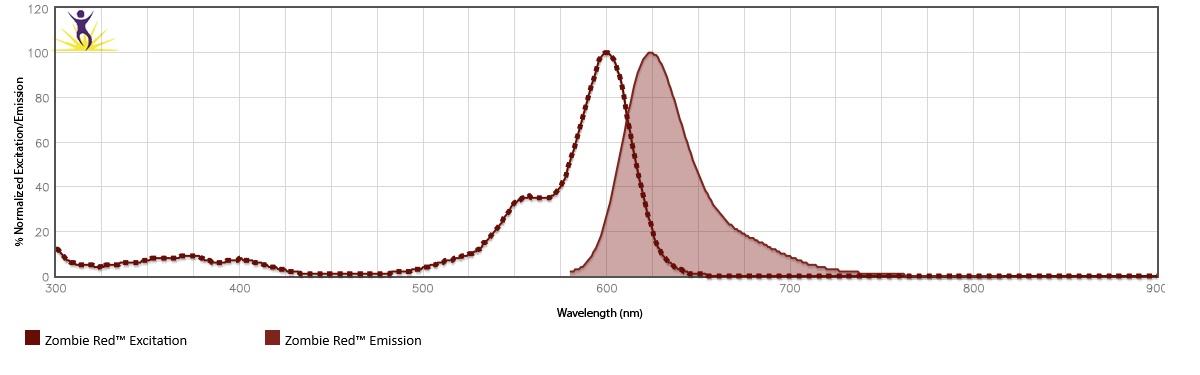
Zombie Red™ is excited by the yellow/green laser (561 nm) and emits maximally at 624 nm. Zombie Red can be weakly excited by the blue laser (488 nm) if a yellow/green laser is not available. This may require a higher concentration of dye to be used along with optimization by the end user.
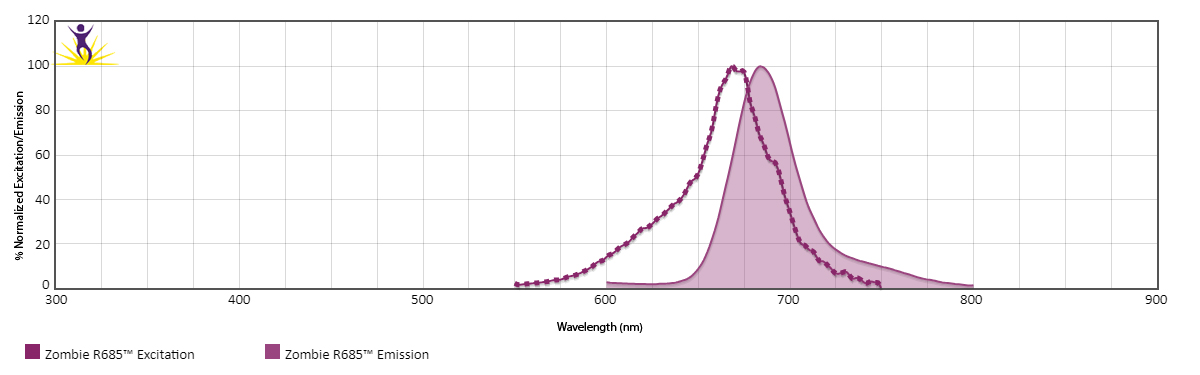
Zombie R685™ is excited by the red laser (633 nm) and emits maximally at 685 nm. It has similar emission spectra to Spark NIR™ 685.
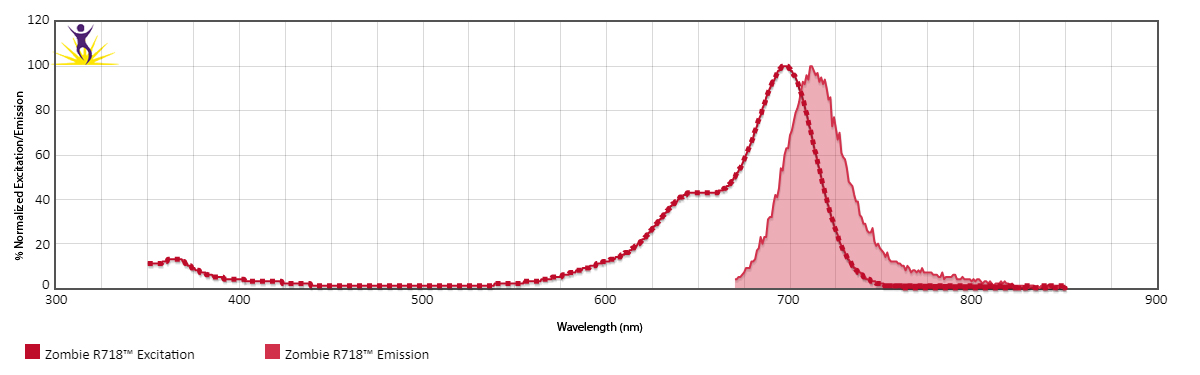
Zombie R718™ is excited by the red laser (633 nm) and emits maximally at 718 nm. It has similar emission spectra to Spark Red™ 718.
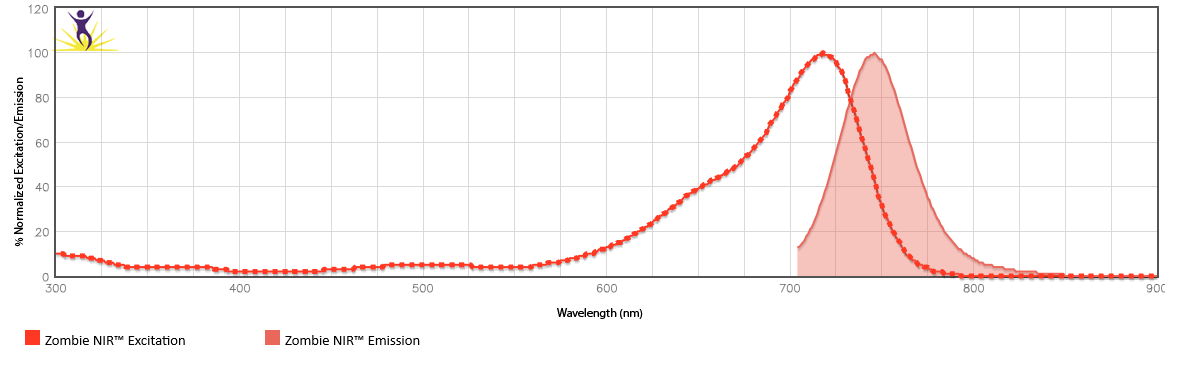
Zombie NIR™ is excited by the red laser (633 nm) and emits maximally at 746 nm.
|
|
Standard Cell Staining Protocol: |
No-wash Sequential Staining Protocol: |
FAQs
Q: Why can’t I fix my cells prior to using Zombie dyes?
A: The fixation process can contort and alter the membrane of cells, effectively rendering them dead. Since the ability of the Zombie dyes to stain dead cells is correlated with cell permeability, your results may no longer be a valid representation of dead versus live cells.
Q: Can I use methanol/ethanol for fixation after using Zombie dyes?
A: Yes, most fixation reagents are fine to be used with Zombie dyes. However, it should be noted that Zombie dyes can still be sensitive to reactive oxygen species. Light exposure or reagents with hydrogen peroxide can lead to free radical formation, affecting fluorescence.
Q: How does the performance of your Zombie dyes compare with competitors?
A: Zombie dyes have been tested against other leading competitors’ fixable viability kits and given comparable results. We also highly recommend that you titrate down the amount of each dye used in order to best match the negative signals of your unstained sample and MFI- (mean fluorescence intensity) stained samples.
Q: Can I use the UV laser to stimulate Zombie Aqua™? If so, can I then use it in conjunction with BV510™?
A: While we typically do not test Zombie Aqua™ with the UV laser, its excitation peak suggests it is effectively excited at 355 nm. However, we would not recommend using BV510™ off the violet laser and Zombie Aqua™ off the UV laser at the same time. Due to cross-beam excitation of BV510™ by the UV laser and the violet excitation of Zombie Aqua™, this would lead to significantly increased background and excessive compensation requirements.
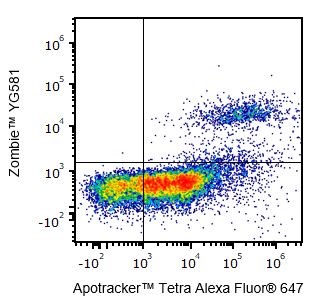
Q: Can I use Zombie dyes to detect apoptotic cells?
A: Yes, Zombie dyes can be used with apoptosis markers, such as Annexin V or Apotracker™ (shown below), to discriminate live, apoptotic, and dead cells.
One day-old C57BL/6 mouse thymocytes were stained with Apotracker™ Tetra Alexa Fluor® 647 and Zombie™ YG581. Zombie-dim/Apotracker™-positive cells are apoptotic, while double-positive cells are dead. Live cells are negative for both markers.
Q: I am concerned about the spillover I am observing from the Zombie dye into its neighboring channels.
A: The rule of thumb with Zombie dyes is to titrate them down as much as possible to fit your application. This should potentially help with spillover. Secondly, Zombie positive events represent dead cells and are typically gated out from analysis.
Q: Can Zombie be used to determine bacteria, yeast viability?
A: We have not tested in house bacterial or yeast viability using Zombie dyes. It is not clear whether the difference between surface and intracellular signals will be significantly different in case of non mammalian cells.
Q: Can I use Zombie with cells suspension containing serum?
A: Serum is full of proteins which will sequester the dye and thereby reducing its effective concentration. The basic rule of thumb with zombie is to titrate it based on your specific condition. Titration also helps reduce the background and spillover into other channels.
Q: Can I use Zombie dyes for microscopy?
A: Zombie dyes tested in-house for microscopy applications will display data on the product technical datasheet. It should be noted that Zombie dyes may not work for dead cell discrimination in every microscopy application. Important considerations that may impact analysis are determining the signal level that constitutes a dead cell and identifying the proper plane to observe the dead cells.
Q: How should I store Zombie dyes?
A: Store the Zombie dye kit at -20°C upon receipt. Do not open vials until needed. Once DMSO is added, use immediately or store at -20°C in a dry place and protected from light, preferably in a desiccator or in a container with desiccant for no more than one month.
 Login / Register
Login / Register 






Follow Us