MojoSort™ Mouse CD335 (NKp46) Selection Kit Column Protocol
Introduction
BioLegend MojoSort™ nanobeads work in commonly used separation columns, based on our internal research as well as validation by external testing by academic labs. This simple protocol consists of following the MojoSort™ protocol to label the cells with pre-diluted MojoSort™ reagents and using the columns as indicated by the manufacturer.
Note: Due to the properties of our beads, it may be possible to use far fewer beads that with other commercial suppliers. If applicable, we provide a suggested dilution factor in the “Application Notes” section of each product webpage based on in-house testing for each kit on columns. However, we still highly recommend performing a titration to find the most optimal dilution factor for your experiment. Please contact BioLegend Technical Service (tech@biolegend.com) if further assistance is needed.
Important Note
MojoSort™ magnetic particles can be used with other commercially available magnetic separators, both free standing magnets and column-based systems. Because MojoSort™ protocols are optimized for the MojoSort™ separator, the protocols may need to be adjusted for other systems. Please contact BioLegend Technical Service (tech@biolegend.com) for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Protocol Steps
- Prepare cells from your tissue of interest or blood without lysing erythrocytes. Kits for human samples have been optimized for PBMCs, please prepare the cells using a suitable method.
- In the final wash of your sample preparation, resuspend the cells in MojoSort™ Buffer by adding up to 4 mL in a 5 mL (12 x 75 mm) polypropylene tube.
Note: Keep MojoSort™ Buffer on ice throughout the procedure. - Filter the cells with a 70 µm cell strainer, centrifuge at 300xg for 5 minutes, and resuspend in an appropriate volume of MojoSort™ Buffer. Count and adjust the cell concentration to 1 x 108 cells/mL.
- Aliquot 100 µL (107 cells) into a new tube. Add 10 µL of the pre-diluted Biotin anti-CD335 (NKp46) antibody. Mix well and incubate on ice for 15 minutes. Scale up the volume if separating more cells. For example, add 100 µL of pre-diluted Antibody for separating 1 x 108 cells in 1 ml of MojoSort™ Buffer. When working with less than 107 cells, use indicated volumes for 107 cells.
- Wash the cells by adding MojoSort™ Buffer up to 4 mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard the supernatant and resuspend cells in 100 µL of MojoSort™ Buffer.
- Vortex the Streptavidin conjugated Nanobeads (to resuspend) at max speed, 5 touches, and prepare the dilutions to test. Add 10 µL of pre-diluted Streptavidin Nanobeads. Mix well and incubate on ice for 15 minutes. Scale up the volume accordingly if separating more cells. For example, add 100 µL of pre-diluted Nanobeads for separating 1 x 108 cells in 1 ml of MojoSort™ Buffer. When working with less than 107 cells, use indicated volumes for 107 cells.
- Wash the cells by adding MojoSort™ Buffer up to 4 mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard the supernatant.
- Add the appropriate amount of MojoSort™ Buffer and proceed to separation. At least 500 µL is needed for column separation.
Note: There are several types of commercially available columns, depending on your application. Choose the one that fits best your experiment:Columns:
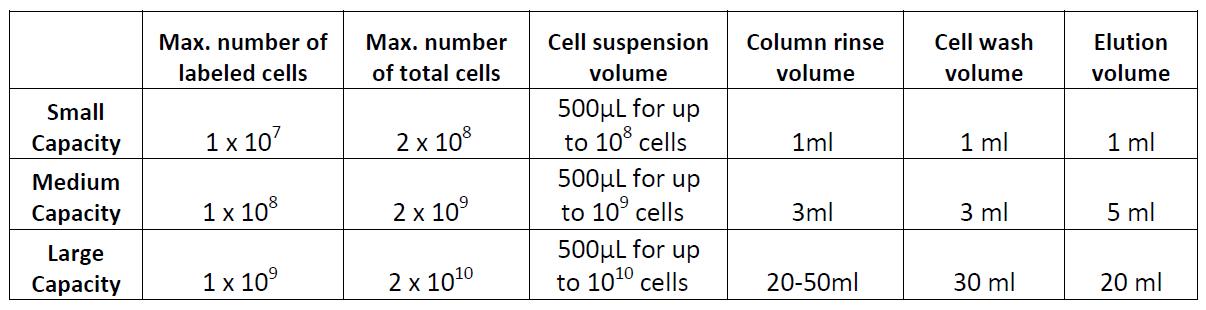
- Place the column in a magnetic separator that fits the column.
- Rinse the column with the appropriate amount of cell separation buffer.
- Add the labeled cell suspension to the column and collect the unlabeled flow through.
- Wash the cells in the column 3 times with the appropriate amount of buffer and collect the fraction unlabeled cells. Combine with the collected fraction from step 13. These cells may be useful as controls, to monitor purity/yield, or other purposes.
- Take away the column from the magnet and place it on a tube. Then add appropriate amount of elution volume and flush out the magnetically labeled fraction with a plunger or supplied device. These are the positively isolated cells of interest; do not discard.
- To increase the purity of the magnetically labeled fraction, harvest the positive fraction (eluted fraction from step 15) and repeat the selection process (steps 10 – 15) with a new, freshly prepared column.
 Login / Register
Login / Register 







Follow Us