- Regulatory Status
- RUO
- Other Names
- VTN, VN, Serum-spreading factor, Complement S-protein, Somatomedin B, V75
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
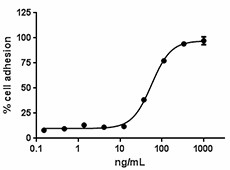
Immobilized Human Vitronectin induces adhesion of HUVEC in a dose dependent manner. ED50 for this effect is 25 - 100 ng/mL.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 757708 | 500 µg | $223 | ||||
Vitronectin was first described as a serum-spreading factor that supported the attachment and spreading of cells onto glass. Vitronectin is a large glycoprotein found in blood and the extracellular matrix. It is a multifunctional protein that mediates cell-to-substrate adhesion, inhibits the cytolytic action of the terminal complement cascade in vitro, and binds to several serine protease inhibitors of the serpin family. Vitronectin is mainly synthesized in the liver and circulates primarily in monomeric form, but can undergo conformational change to a structure that forms disulfide linked multimers. The vitronectin molecule contains Somatomedin-B domain (SMB), four hemopexin domains, and the carboxyl-terminal end which has multiple sites and functions. The SMB domain mediates interaction with Serpine1/PAI1 and the heparin-binding domain at the carboxyl-terminal end mediates interaction with insulin. The carboxyl-terminal end also contains a plasminogen binding site, a glycosaminoglycan binding site, and a second PAI-1 binding site. The heparin binding site also mediates complement factor C7, C8, or C9 binding. Vitronectin’s ablility to bind platelet glycoproteins and mediate platelet adhesion and aggregation at sites of vascular injuries has made it an important mediator in the pathogenesis of coronary atherosclerosis.
Product DetailsProduct Details
- Source
- Human Vitronectin, amino acids Asp20-Leu478 (Accession# AAH05046.1), was expressed in HEK293.
- Molecular Mass
- The 459 amino acid recombinant protein has a predicted molecular mass of approximately 52.3 kD. The protein migrates at an apparent molecular weight of 75 kD by SDS-PAGE under reducing conditions. The predicted N-terminal amino acid is Asp.
- Purity
- >95%, as determined by SDS-PAGE gel and HPLC analysis.
- Formulation
- Lyophilized from 0.2 µm filtered protein solution in 20 mM Sodium Phosphate, pH 7.5.
- Endotoxin Level
- Less than 1 EU per µg protein as determined by the LAL method.
- Storage & Handling
- Unopened vial can be stored at -20°C or -70°C. For maximum results, quick spin vial prior to opening. Reconstitute in water to a concentration of 0.1-0.5 mg/ml. Do not vortex. It is recommended to further dilute in a buffer containing a carrier protein such as 0.1% BSA and store working aliquots at -20°C to -80°C. Avoid repeated freeze/thaw cycles.
- Activity
- ED50 = 25 - 100 ng/mL as measured by the ability of immobilized protein to support the adhesion of HUVEC.
- Application
-
Bioassay
- Product Citations
-
Antigen Details
- Structure
- Monomer and Multimer.
- Distribution
-
Mainly synthesized in the liver, high concentration in vasculature, and is found in most tissues.
- Function
- Mediates cell-to-substrate adhesion, inhibits the cytolytic action of the terminal complement cascade in vitro, and binds to several serine protease inhibitors of the serpin family.
- Interaction
- Interacts with SERPINE1/PAI1, insulin, C1QBP, and extracelluar matrix.
- Ligand/Receptor
- SERPINE1/PAI1, insulin, C1QBP, and Integrins.
- Bioactivity
- Measured by the ability of immobilized human Vitronectin to induce adhesion of HUVEC.
- Cell Type
- Embryonic Stem Cells
- Biology Area
- Complement, Stem Cells
- Antigen References
-
1. Jenne D, et al. 1985. EMBO J. 4:3153-3157.
2. Conlan MG, et al. 1988. Blood 72:185-190.
3. Sun WH, Mosher DF. 1989. Blood 73:353-354.
4. Sigurdardottir O, Wiman B. 1994. Biochim. Biophys. Acta. 1208:104-110.
5. Yaoi Y, et al. 1991. Exp. Cell Res. 194: 180-185.
6. Seiffert D, Loskutoff DJ. 1991. J. Biol. Chem. 266:2824-2830.
7. Lim BL, et al. 1996. J. Biol. Chem. 271:26739-26744.
8. Zhou A, et al. 2003. Nat. Struct. Biol. 10:541-544.
9. Ekmekci OB, et al. 2006. Clin. Chim. Acta. 368(1-2):77-83.
10. Derer W, et al. 2009. Circ. Cardiovasc. Interv. 2(1):14-9.
11. Heyman L, et al. 2010. Cell Biol. Int. 34(5):493-502. - Gene ID
- 7448 View all products for this Gene ID
- UniProt
- View information about Vitronectin on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login/Register
Login/Register 








Follow Us