MojoSort™ Streptavidin Nanobeads Column Protocol - Negative Selection
Introduction
BioLegend MojoSort™ nanobeads work in commonly used separation columns, based on our internal research as well as validation by external testing by academic labs. This simple protocol consists of following the MojoSort™ protocol to label the cells with pre-diluted MojoSort™ reagents and using the columns as indicated by the manufacturer.
Note: Due to the properties of our beads, it may be possible to use far fewer beads and less antibody cocktail that with other commercial suppliers. We recommend a titration to find the best dilution factor. However, as a general rule, dilutions ranging from 1:2 to 1:10 for the antibody cocktail can be used. Dilutions ranging from 1:5 to 1:20 for the Streptavidin Nanobeads can be used. Please contact BioLegend Technical Service (tech@biolegend.com) if further assistance is needed.
Important Note
MojoSort™ magnetic particles can be used with other commercially available magnetic separators, both free standing magnets and column-based systems. Because MojoSort™ protocols are optimized for the MojoSort™ separator, the protocols may need to be adjusted for other systems. Please contact BioLegend Technical Service (tech@biolegend.com) for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Protocol Selection: If your target cells are the labeled cells (the positive fraction), use the Streptavidin Nanobeads Column Protocol – Positive Selection. If your target cells are the unlabeled cells (negative fraction), use the Streptavidin Nanobeads Column Protocol - Negative Selection.
Protocol Steps
- Prepare cells from your tissue of interest or blood without lysing erythrocytes.
- In the final wash of your sample preparation, resuspend the cells in MojoSort™ Buffer by adding up to 4 mL in a 5 mL (12 x 75 mm) polypropylene tube.
Note: Keep MojoSort™ Buffer on ice throughout the procedure. - Filter the cells with a 70 µm cell strainer, centrifuge at 300xg for 5 minutes, and resuspend in an appropriate volume of MojoSort™ Buffer. Count and adjust the cell concentration to 1 x 108 cells/mL by adding MojoSort™ Buffer.
- Aliquot 100 µL of cell suspension (107 cells) into a new tube. Check the recommended usage for flow cytometric staining of the Biotin-conjugated antibody indicated in the antibody technical datasheet. Calculate the volume to stain 107 cells (or desired amount of cells). Add the appropriate volume of pre-diluted Biotin-conjugated antibody to the cell suspension, mix well and incubate on ice for 15 minutes.
Note: For the Biotin-conjugated antibodies, we recommend to do a titration to determine the optimal concentration. - Wash the cells by adding MojoSort™ Buffer up to 4 mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard the supernatant and resuspend cells in 100 µL of MojoSort™ Buffer.
- Resuspend the beads by vortexing, maximum speed, 5 touches. Add the appropriate volume of pre-diluted Streptavidin Nanobeads. Mix well and incubate on ice for 15 minutes. Scale up the volume accordingly if separating more cells. For example, if the volume of pre-diluted Nanobeads for 1x107 cells is 10 µL, add 100 µL for 1 x 108 cells. When working with less than 107 cells, use indicated volumes for 107 cells.
Note: The amount of Nanobeads to use always depends on the frequency of the target, among a few other factors. We recommend to do a titration to determine the optimal concentration. - Wash the cells by adding MojoSort™ Buffer up to 4 mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard the supernatant.
- Resuspend the cells in appropriate amount of MojoSort™ Buffer and proceed to separation. At least 500 µL is needed for column separation.
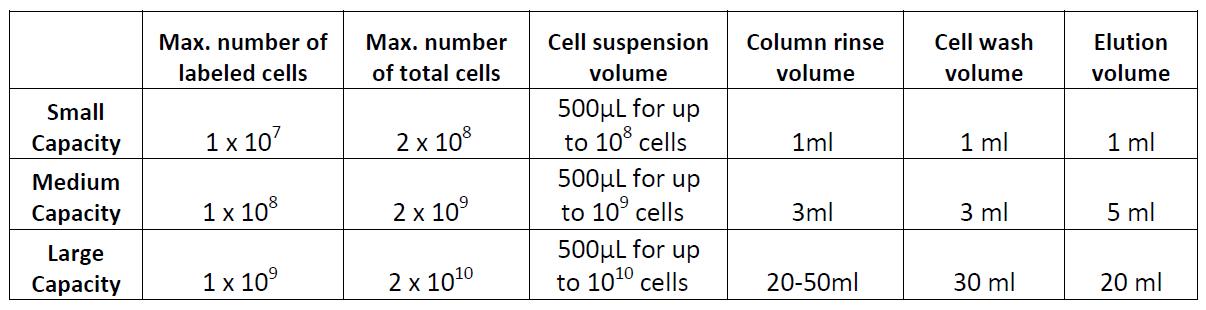
Note: There are several types of commercially available columns, depending on your application. Choose the one that fits best your experiment:
Columns:
Example of magnetic separation with medium capacity columns:
- Place the column in a magnetic separator that fits the column.
- Rinse the column with 3 mL of cell separation buffer.
- Add the labeled cell suspension in at least 500 µL of buffer to the column through a 30 µm filter and collect the fraction containing the unlabeled cells. These are the cells of interest; do not discard.
- Wash the cells in the column 2 times with 3 mL of buffer and collect the fraction containing the untouched cells. Combine with the collected fraction from step 3.
- If desired, the labeled cells can be collected by taking away the column from the magnet and place it on a tube. Then add 5 mL of buffer and flush out the magnetically labeled fraction with a plunger or supplied device. The labeled cells may be useful as staining controls, to monitor purity/yield, or other purposes.
Data
Flow cytometry. High purity and yield. “After Isolation” plot shows purified population of interest using pre-diluted MojoSort™ reagents in separation columns.
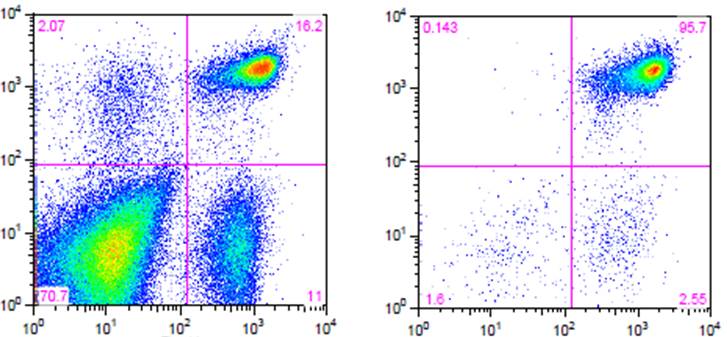
| Kit | Purity | Yield |
|---|---|---|
| Mouse CD4 T Cell Isolation Kit | 95.7% | 85% |
Electron Microscopy. CD4+ T cells Isolated with MojoSort™ CD4 T Cell Isolation Kit using columns do not display particles in the cell surface. Image is representative of 36 different cells.
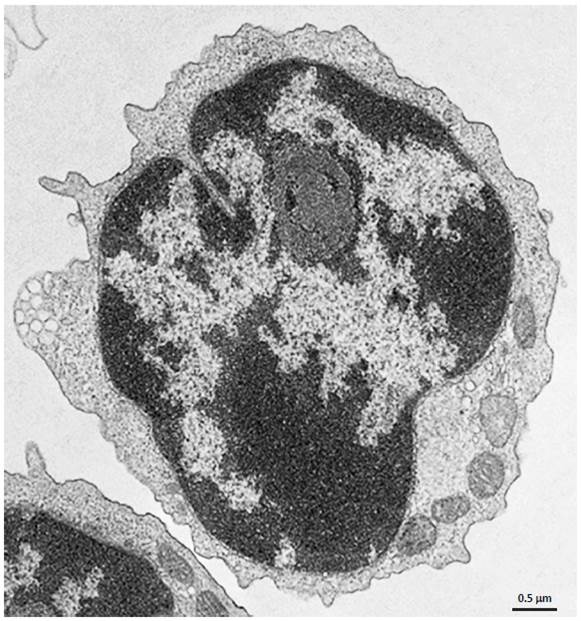 CD4+ T cells isolated with MojoSort™ CD4 T Cell Isolation Kit using separation columns. |
 Login/Register
Login/Register 







Follow Us