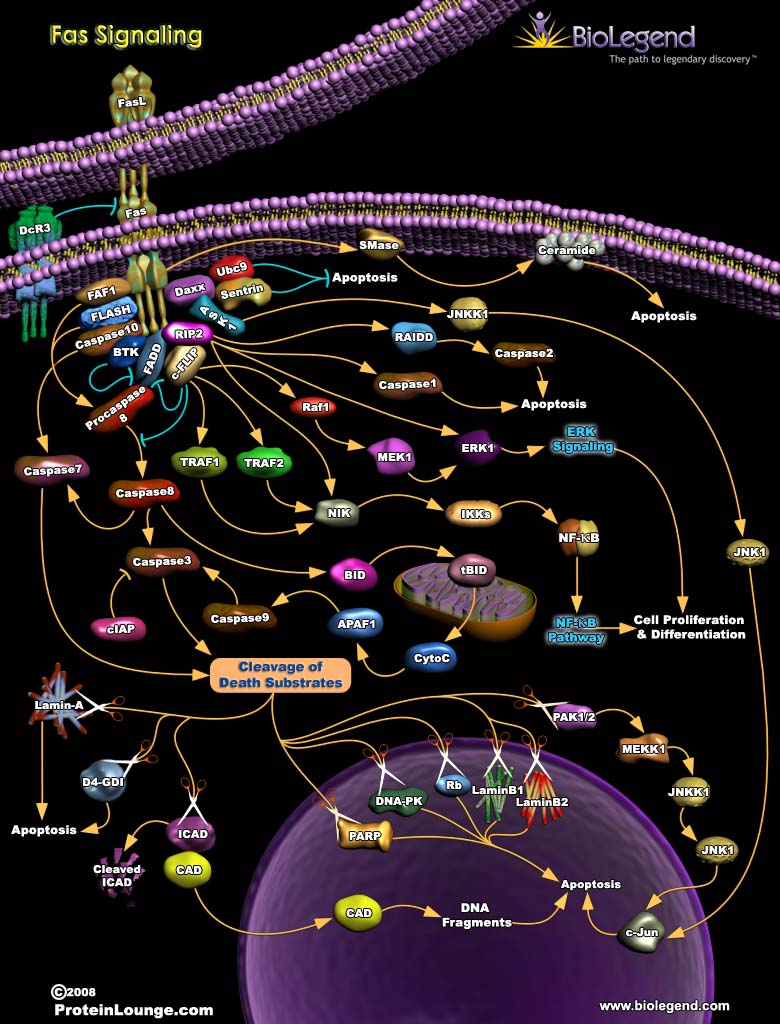
Fas Signaling
Fas receptors are death receptors activated by Fas ligand (FasL) expressed on immune cells like cytotoxic T cells and NK cells. When bound by FasL, Fas receptors initiate signaling through their cytoplasmic death domains to induce the apoptosis pathway. This involves recruitment and activation of procaspase 8 by the protein FADD, and cleavage of caspase 3 and caspase 7. These caspases go on to cleave their death substrates, including Rb and PARP, causing cell death in type I cells like thymocytes. In type II cells like B cells, caspase 8 cleaves BID, which mediates the release of cytochrome c from mitochondrial membranes, resulting in apoptosome formation and caspase 9 activation. Caspase 9 goes on to trigger other caspase cleavage events that lead to apoptosis. Fas signaling can also cause FADD to recruit c-FLIP, an inhibitor of caspase 8, which will facilitate the activation of NF-κB, promoting cell survival and proliferation. In contrast to membrane-bound FasL, soluble FasL inhibits apoptosis and promotes cell growth through the ERK1 signaling pathway.
Click on the poster below to view the interactive version.
 Login/Register
Login/Register 







Follow Us