Loading...
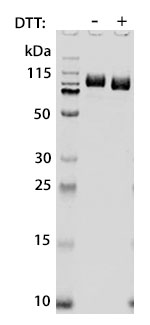
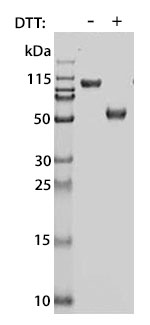
Prior to performing bioassay validation and stability testing, we test each recombinant protein for product purity and quantification. In the table below you can find examples of the attributes tested, the test performed, and some acceptance criteria used to ensure that our customers are confident they are receiving the highest quality reagents.
| Parameter | Testing |
|---|---|
|
Purity |
SDS-PAGE, FPLC, (>95%) |
|
Quantity |
UV Spec, SDS-PAGE |
|
Molecular Weight |
DTT-reduced and non-reduced SDS-PAGE |
|
Microbiological Control |
0.2 μm Filtration |
|
Endotoxin Level |
LAL Assay (<0.1 ng/μg protein or <1 EU/μg protein) |
ProductsHere
 Login/Register
Login/Register 










Follow Us