- Regulatory Status
- RUO
- Other Names
- FAP, FAP-alpha, Fibroblast Activation Protein alpha, fibroblast activation protein, alpha, Integral membrane serine protease, Seprase, DPP4
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
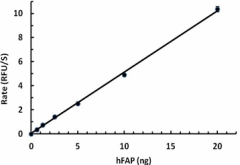
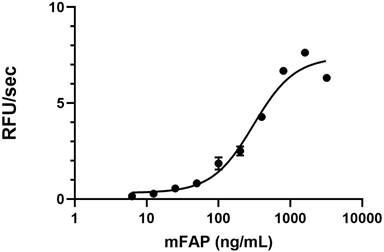
The activity of human FAP was measured with 50 µM of fluorogenic substrate, Z-GP-AMC, in the presence of 0.625, 1.25, 2.5, 5, 10, and 20 ng of human FAP. The activity of hFAP is greater than 4700 pmole/min/µg. -
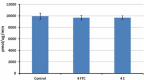
The aliquots of human FAP were treated at three different conditions: one week at -20°C (Control), four freeze-and-thaw cycles (FTC), and one week at 4°C (4 C). Human FAP is stable at all of the conditions tested compared to the control.
Fibroblast activation protein α (FAP), also known as seprase, is a type II transmembrane serine protease that can be shedded into plasma. FAP is a proline specific enzyme with a preference of Proline as P1 residue. FAP and a dipeptidyl peptidase 4 (DPP4) shares similar structural fold, featuring an α/β-hydrolase domain and an eight bladed β-propeller domain. Known DPP4 dipeptides are cleaved by FAP with an 100- fold decrease in catalytic efficiency compared with DPP4. In addition to dipeptidyl peptidase activity, it possesses endopeptidase activity capable of cleaving gelatin and type I collagen. FAP is expressed in the majority of epithelial cancer-associated fibroblasts, in granulation tissue of healing wounds, and in bone and soft tissue sarcomas. Expression of FAP is not detected in fibroblasts of benign epithelial tumors or normal adult tissues. It has been implicated in tumor growth and metastasis, and in extracellular matrix remodeling. The shedded FAP in plasma can cleave α-2-antiplasmin (Serpin F2) into a more active form.
Product DetailsProduct Details
- Source
- Human FAP, amino acids Arg27-Asp760 (Accession # NM_004460) with a C-terminal TG-8H-GGQ tag was expressed in CHO cells.
- Molecular Mass
- The 747 amino acid recombinant protein has a predicted molecular mass of approximately 86.4 kD. The non-reduced and DTT-reduced proteins migrate at 80 - 90 kDa by SDS-PAGE.
- Purity
- > 95%, as determined by Coomasie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in 16 mM Tris, 240 mM NaCl, and 20% glycerol.
- Endotoxin Level
- Less than 1.0 EU per µg of protein as determined by the LAL method.
- Concentration
- 10-25 µg sizes are bottled at 200 µg/mL. 100 µg and larger sizes and larger are bottled at the concentration indicated on the vial.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for one month, at -20°C for three months, or at -70°C for six months. For maximum results, quick spin vial prior to opening. Avoid repeated freeze/thaw cycles.
- Activity
- Human FAP cleaves a fluorogenic substrate Z-Gly-Pro-AMC with a specific activity value > 4700 pmol/µg/min.
- Application
-
Bioassay
- Application Notes
-
Human FAP Activity assay
Human FAP activity is measured by its ability to cleave a fluorogenic peptide substrate Z-Gly-Pro-AMC. The increase of the product formation is monitored by increase in intensity of fluorescence at 460 nm with excitation at 380 nm.
Materials
- Assay Buffer: pH 7.5, 50 mM Tris, 1.0 M NaCl, 0.1% BSA (w/v)
- Recombinant Human FAP
- FAP substrate: Z-Gly-Pro-AMC
Activity assay procedures
- Dilute hFAP in the assay buffer at 0.2 µg/mL.
- Dilute the substrate at 100 µM in Assay Buffer.
- Load into a well plate 50 µL of the 0.2 ng/µL hFAP and start the reaction by adding 50 µL of 100 µM Substrate. Include a substrate blank containing 50 µL of Assay Buffer and 50 µL of 100 µM Substrate solution.
- Read the product formation by measuring 380/460 nm (Excitation/Emission) in kinetic mode for 5 minutes.
- The final hFAP concentration is 0.01 µg/100 µL reaction.
- The substrate is 50 µM.
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com. - Application References
-
- Lee KN, et al. 2006. Blood 107:1397.
- Waumans Y. et al. 2015. Front Immunol. 6:387.
- Kotackovß L, et al. 2009. Folia Biol. (Praha) 55(3):77-84.
- Sulda ML, et al. 2006. 575:197.
- Aertgeerts K, et al. 2005. J. Biol. Chem. 280:19441.
Antigen Details
- Structure
- Monomer form
- Distribution
-
Adipose tissue and fibrobasts
- Function
- Tissue remodeling, fibrosis, wound healing, inflammation and tumor growth and metastasis.
- Interaction
- Serpin F2 (alpha-2-antiplasmin); Gelatin (Type I collagen)
- Biology Area
- Cancer Biomarkers
- Molecular Family
- Enzymes and Regulators
- Gene ID
- 2191 View all products for this Gene ID
- UniProt
- View information about FAP on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login / Register
Login / Register 








Follow Us