- Regulatory Status
- RUO
- Other Names
- CTSL, Major Excreted Protein (MEP), CATL
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
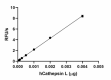
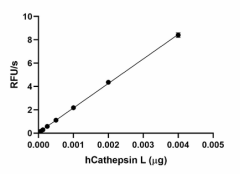
Recombinant human Cathepsin L cleaves fluorogenic peptide substrate Z-LR-AMC. The accumulation of cleavage product is measured by an increase in excitation and emission at 380 and 460 nm, respectively. The recommended specific activity is > 23,000 pmol/min/µg. -
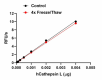
Stability Testing for Recombinant Human Cathepsin L. Recombinant human Cathepsin L was aliquoted in 50 mM sodium acetate, 0.1% glacial acetic acid, 0.1 M NaCl, 40% glycerol, pH 5 at 0.2 mg/mL and one aliquot was kept at 4°C (Control), and another was frozen and thawed four times (4x Freeze/Thaw). After this procedure, the samples were tested for their ability to cleave Z-LR-AMC. The recommended specific activity is > 23,000 pmol/min/µg.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 787504 | 25 µg | 259€ | ||||
| 787506 | 100 µg | 746€ | ||||
Cathepsin L, a member of the peptidase C1 family, plays an important role in intracellular protein catabolism and antigen processing (1). It is a cysteine protease that consists of a dimer composed of difulside-linked heavy and light chains originating from a single precursor (2). The mature form is characterized by the removal of an N-terminal pro-peptide. Substrates of the active enzyme include collagen and elastin, making it a relevant disesase marker for atherosclerosis and cancers (3). Cathepsin L also participates in autophagy; it degrades two autophagosomal markers, LC3-II and GABARAP-II. Active cathepsin L degrades two key proteins in podocytes, GTPase dynamin and synaptopodin required for the proper glomerular filtration function. Cathepsin L expression is dysregulates in human diseases, including liver fibrosis, abdominal aortic aneurysm, diabetes, and cancer. Since it is able to cleave the S1 subunit of the SARS-CoV-2 spike protein, Cathepsin L may be essential for SARS-CoV-2 viral entry (4). In addition, Cathepsin L and heparanase promote herpes simplex virus 2 release from infected cells.
Product DetailsProduct Details
- Source
- Human Cathepsin L, amino acid (Thr18-Val333) (Accession: P07711), with a C-terminal 8His tag, was expressed in 293E cells.
- Molecular Mass
- The 327 amino acid recombinant protein has a predicted molecular mass of approximately 25.5 kD. The protein migrates at approximately 31 kD and 27 kD by SDS-PAGE in DTT-reduced and non-reduced conditions, respectively. The predicted N-terminal amino acid is Thr.
- Purity
- > 96%, as determined by Coomassie stained SDS-PAGE
- Formulation
- 0.22 µm filtered protein solution is in 50 mM sodium acetate, 0.1% glacial acetic acid. 0.1 M NaCl, 40% glycerol, pH 5.
- Endotoxin Level
- Less than 0.1 EU per µg cytokine as determined by the LAL method
- Concentration
- Lot-specific (to obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.)
- Storage & Handling
- Unopened vial can be stored at -20°C or -70°C for six months. For maximum results, quick spin vial prior to opening. Avoid repeated freeze/thaw cycles.
- Activity
- Recombinant human Cathepsin L is measured by its ability to cleave Z-LR-AMC. Cleavage product is detected by an increase in fluorescence at excitation and emission at 380 and 460 nm, respectively. The recommended specific activity is > 23,000 pmol/min/µg.
- Application
-
Bioassay
- Application Notes
-
Human Cathepsin L Assay Procedure
Human Cathepsin L activity is measured by its ability to cleave the fluorogenic peptide substrate Z-LR-AMC. The accumulation of cleavage product is quantified by the increase in fluorescence at 460 nm upon excitation at 380 nm.
Materials and Buffers
1. Assay Buffer: 50 mM MES, 5 mM DTT, 1 mM EDTA, 0.005% (w/v) Brij-35, pH 6.02. Human CTSL substrate: Z-LR-AMC (Bachem Cat. No. I-1960)3. Recombinant human cathepsin L4. F16 Black Maxisorp Plate (Nunc, Cat. No. 475515)5. Fluorescent Plate Reader (Model: SpectraMax Molecular Devices) or equivalent
Assay Procedure
1. Dilute the human CTSL to 40 µg/mL in assay buffer.2. Incubate the diluted human CTSL on ice for 15 minutes.3. Dilute the human CTSL to 0.08 ng/µL in assay buffer. Make six 1:2 dilutions in a black well plate. Include a blank with 50 µL assay buffer.4. Dilute the substrate to 80 µM in assay buffer.5. Start the reaction by adding 50 µL of the diluted substrate to each well.6. Read at excitation and emission wavelengths of 380 nm and 460 nm (top read), respectively, in kinetic mode for 5 minutes.7. Calculate specific activity:
Specific Activity (pmol/min/µg) = Adjusted Vmax* (RFU/min) x Conversion Factor** (pmol/RFU)
amount of enzyme (µg)*Adjusted for Substrate Blank
**Derived using calibration standard 7-Amino, 4-Methyl Coumarin (AMC) (Sigma, Catalog # A-9891).
Per well:• Human CTSL: 4, 2, 1, 0.5, 0.25, 0.125, and 0.0625 ng• Substrate: 40 µM - Additional Product Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are validated in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Structure
- Enzyme endopeptidase
- Distribution
-
Ubiquitously expressed endopeptidase, highly expressed in macrophages
- Function
- Intracellular protein catabolism, participates in autophagy, controls release of trypsinogen from pancreatic cells and activation in duodenum, regulates secretory vesicles in the neuroendocrine system, homeostasis is cardiomyocytes. Activated and mature cathepsin L are regulated by cystatins, endogenous inhibitors.
- Interaction
- Substrates LC3-II, GABARAP-II, GTPase dynamin, and synaptopodin.
- Ligand/Receptor
- Peptide bonds with aromatic amino acids (aa) in P2 and hydrophobic aa in P3 position
- Bioactivity
- Recombinant Human Cathepsin L cleaves fluorogenic peptide substrate Z-LR-AMC.
- Cell Type
- Endothelial cells, Epithelial cells, Fibroblasts, Macrophages, Monocytes, T cells
- Biology Area
- Cancer Biomarkers, Cardiovascular Biology, Cell Biology, Cell Proliferation and Viability, COVID-19, Immuno-Oncology, Neuroscience
- Molecular Family
- Enzymes and Regulators, Lysosomal Markers, Proteases
- Antigen References
-
- Creasy BM & McCoy KL. 2011. Cell Immunol. 267:56.
- Coulombe R, et al. 1996. EMBO J. 15:5492.
- Sudhan DR & Siemann DW, 2015. Pharmacol Ther. 155:105.
- Katopodis, P, et al. 2020. Int J Oncol. 57:533.
- Hopkins JN, et al. 2018. J Virol. 92:e01179.
- Ueno T. & Takahashi K. 2009. Autophagy. 5:878
- Dana D & Pathak SK. 2020. Molecules 25:698.
- Gene ID
- 1514 View all products for this Gene ID
- UniProt
- View information about Cathepsin L on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)
 Login / Register
Login / Register 








Follow Us