Complement System
The complement system, also known as the complement cascade, comprises a series of around 20 different proteins that circulate freely in the blood and tissue fluids. While the complement proteins are usually inactive, they sequentially activate one another upon encountering a foreign micro-organism to enhance – or complement – other aspects of the innate immune response.
Activation of the complement system occurs via three main pathways: classical, mannose binding lectin (MBL), and alternative. The classical pathway is triggered by antibody-antigen immune complexes binding to the complement protein C1, which circulates as a complex of six C1q molecules and two molecules each of the serine proteases C1r and C1s. Following interaction between the fragment crystallizable (Fc) portion of the antibody and C1q, the resulting conformational change activates C1r and C1s, which in turn cleave the complement proteins C2 and C4 to generate the C3 convertase C4bC2a. This cleaves C3, the central component of the complement cascade, forming the effector molecules C3a and C3b. In turn, C3b can complex with C4bC2a to form the C5 convertase C4bC2aC3b; this cleaves C5 to form C5b, a key component of the membrane attack complex (MAC).
The MBL pathway is similar to the classical pathway in that it also leads to the formation of C4bC2a. However, it is initiated by mannose residues present on the surface of micro-organisms such as bacteria and fungi. Binding of circulating MBL to these residues activates the MBL-associated serine proteases MASP-1, MASP-2 and MASP-3, which cleave C2 and C4 to generate C4bC2a.
A defining feature of the alternative pathway is that it is constitutively active at low levels, ensuring that the complement system is constantly primed for rapid activation. Tightly controlled hydrolysis of a thioester bond within C3 is thought to trigger the alternative pathway, which is often referred to as ‘the amplification loop’ because it generates another C3 convertase (C3bBb) that can activate additional C3 to that activated via the classical and MBL pathways.
Activated complement gives rise to three main types of effector molecules. The first of these are the anaphylatoxins (C3a and C5a), which attract leukocytes to the site of infection and activate them through interaction with the G-protein–coupled receptors (C3aR and C5aR) expressed at the leukocyte surface. Opsonins (C3b, iC3b, and C3d) are a second group of effector molecules, which instead bind to foreign pathogens or infected self-cells, targeting them for phagocytosis. Lastly, the MAC forms pores in the membrane of pathogens or targeted cells, resulting in their destruction by osmolysis.
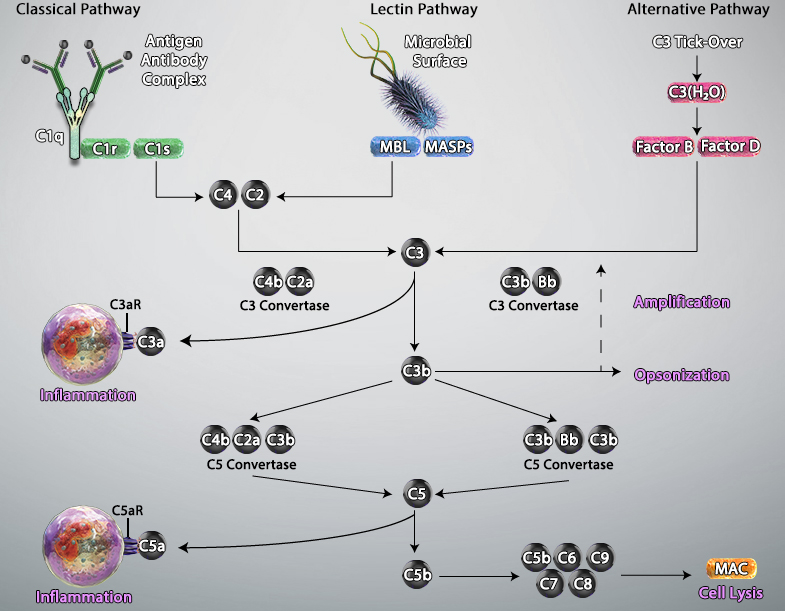
Adapted from Trouw, Leendert A et al. “The complement system as a potential therapeutic target in rheumatic disease.” Nature reviews. Rheumatology vol. 13,9 (2017): 538-547. doi:10.1038/nrrheum.2017.125
 Login / Register
Login / Register 







Follow Us