Discover the Versatility of iPSCs in Parkinson’s Disease Modeling
Discovery of new treatments for Parkinson’s disease (PD) requires mechanistic studies using disease models that accurately reproduce PD characteristics. Visualizing pathology, such as Lewy body layers in primary brain tissues, allows us to describe important phenotypes. However, disease models are needed to explain their underlying causes. Knowing the mechanisms that drive disease can help propel therapeutic development.
Using iPSCs to Understand Parkinson’s Disease
Building PD models has been challenging, due to the limited access to human neuronal tissues and the inability of animals to mirror human disease features. Faced with these hurdles, researchers now look to a new tool for creating advanced PD models: induced pluripotent stem cells (iPSCs) [1]. By manipulating the expression of transcription factors like SOX2, Oct3/4, c-Myc, and Klf4, somatic cells can be converted into iPSCs, which can then be differentiated into other cell types of interest. This allows researchers to reprogram easily obtainable cells, like skin or blood cells, into cell types that are more rare and inaccessible, such as neurons. In this blog post, we discuss how recent advancements in disease modeling with iPSC-derived neurons is deepening our understanding of mechanisms behind PD pathogenesis.
Parkin and PINK1 Mutations Disturb Sleep Patterns
PINK1, a serine/threonine kinase, and Parkin, a ubiquitin ligase with neuroprotective effects, work together to orchestrate mitochondrial maintenance and mitophagy in neurons. Though mutations in PINK1 and Parkin are typically associated with familial (heritable) Parkinson’s, loss of Parkin function also occurs with sporadic PD. A shared symptom of familial and sporadic PD that often precedes motor dysfunction is sleep pattern disturbance, which can result in insomnia and loss of circadian rhythms. This led researchers to investigate the link between Parkin and PINK1 function and sleep-related illnesses during PD. In a paper published in Neuron, Valadas et al. studied iPSC-derived neurons from PD patients carrying Parkin or PINK1 mutations. They found that neurons with these mutations had depleted levels of phosphatidylserine in the endoplasmic reticulum, which caused a decrease in the production of neuropeptide-containing vesicles important
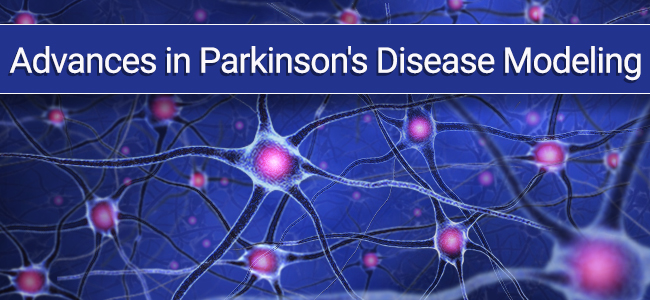
IHC staining with purified anti-Parkin antibody (clone A15165E) on formalin-fixed paraffin-embedded Parkinson’s disease substantia nigra tissue. BioLegend’s Ultra Streptavidin (USA) HRP Detection Kit (Multi-Species, AEC, Cat. No. 929501) was used for detection, followed by hematoxylin counterstaining.
for sleep regulation.This suggested that decreased phosphatidylserine was the cause of sleep disturbance in PD patients. When the researchers tested this theory in fruit flies that harbored Parkin and PINK1 mutations, they found that supplementing the flies’ diet with phosphatidylserine could cure their sleep defects. Further disease modeling research may help determine whether this pathway can be targeted to treat non-motor symptoms of PD.
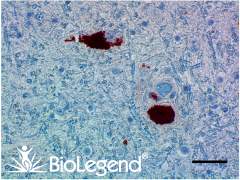
IHC staining with purified anti-α-Synuclein, aggregated antibody (clone A17183G) on formalin-fixed paraffin-embedded Parkinson’s disease brain tissue. Following staining with primary antibody overnight at 4°C, tissues were incubated with Alexa Fluor® 647 goat anti-rat IgG (Cat. No. 405416) for one hour at room temperature. Nuclei were counterstained with DAPI.
Alpha-Synuclein Aggregates Induce Mitochondrial Permeability
The SNCA gene encodes α-Synuclein, a protein with numerous roles in neuronal tissues, including regulation of ATP synthesis by mitochondria [3]. A hallmark of Parkinson’s disease is the formation of α-Synuclein aggregates within inclusions called Lewy bodies. Though it is known that these aggregates drive Lewy body pathology in the brain during PD, it is less clear how they directly affect mitochondria. In a study by Ludtmann et al. published in Nature Communications [4], researchers found that α-Synuclein aggregates cause dysregulation of mitochondrial membrane permeability. To demonstrate this, the scientists used iPSC-derived neurons generated from a patient who had an SNCA gene triplication – one type of genetic mutation that can cause autosomal dominant early-onset PD. Compared to control cells, neurons with SNCA triplication had increased α-Synuclein aggregate staining and higher levels of NADH buildup, an indicator of electron transport chain disruption.
Further experimentation using mitochondrial labeling and stimulation showed that α-Synuclein aggregates caused the irregular opening of pores in the mitochondrial membrane, which led to mitochondrial dysfunction and cell death. Using iPSCs in these experiments was critical for showing the mechanisms by which real genetic causes of PD impact mitochondrial function. These results may therefore inform the design of therapies aimed at preventing mitochondrial dysfunction or α-Synuclein aggregation in patients with autosomal dominant PD.
Modeling LRRK2 Mutations in 3D Organoids
The most common cause of late-onset familial and sporadic PD is the presence of mutations in the gene encoding the LRRK2 protein, a leucine-rich repeat kinase. Modeling these mutations in the lab has been particularly challenging, since animals with LRRK2 mutations do not clearly display Lewy body formation or progressive dopamine neuron loss. Even when using iPSC-derived neurons that have a mutated LRRK2 gene, it has been difficult for researchers to recapitulate the severity of disease observed in actual human brain tissues. Could the inability of iPSC-derived neurons to mimic LRRK2-associated disease be due to the structural immaturity of 2D cell cultures? In an effort to build improved modeling for LRRK2 mutations, Kim et al. (Stem Cell Reports 2019) generated 3D midbrain organoids with and without the PD-associated G2019S LRRK2 mutation [5]. To construct these organoids, researchers organized iPSCs into 3D aggregates, called embryoid bodies, and cultured them on Matrigel® – a protein mixture that resembles the extracellular composition of biological tissues. These cultures were treated with a combination of inhibitors,
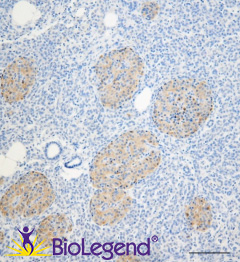
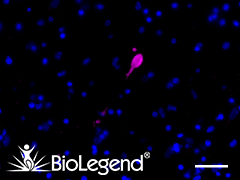
IHC staining with purified anti-LRRK2 antibody (clone MC.028.83.76.242) on formalin-fixed paraffin-embedded human pancreas tissue. BioLegend´s Ultra-Streptavidin (USA) HRP kit (Multi-Species, DAB, Cat. No. 929501) was used for detection, followed by hematoxylin counterstaining.
growth factors, and neurotrophic factors to induce proper differentiation of brain cells. When midbrain organoids matured, the researchers observed that mutant organoids reproduced PD-associated α-Synuclein aggregates. The researchers then looked for gene expression differences between healthy and LRRK2 mutant organoids, and they found that mutants contained higher expression levels of TXNIP, a thiol-oxidoreductase previously identified as a PD risk factor. Knockdown of TXNIP in the mutant organoids showed a reduction in α-Synuclein aggregates, indicating that TXNIP was a driver of disease. This study demonstrates how iPSCs can be utilized to generate even more advanced models like 3D brain organoids, which are capable of elucidating disease mechanisms that could not be captured previously.
In support of Parkinson’s research, we are offering a 50% discount (until May 31, 2020) on our anti-α-Synuclein, anti-Parkin, and anti-PINK1 antibodies that can be used in disease modeling research:
| Specificity | Clone | Reactivity | Composition |
|---|---|---|---|
| α-Synuclein (C-Terminal Truncated x-122) |
A15127A | Human | IHC-P, ELISA |
| α-Synuclein, 117-122 | A15126D | Human | IHC-P, WB, ELISA |
| α-Synuclein, 34-45 | A15110D | Human | IHC-P, ELISA |
| α-Synuclein, 80-96 | A15115A | Human, Mouse, Rat | IHC-P, WB, ELISA |
| α-Synuclein, aggregated | A17183A | Human | IHC-P |
| α-Synuclein, aggregated | A17183B | Human | IHC-P |
| α-Synuclein, aggregated | A17183G | Human | IHC-P |
| α-Synuclein Phospho (Ser129) | P-syn/81A | Human | IHC-P, ICC, WB |
| Parkin | 5C3/Parkin | Human, Mouse, Rat | WB |
| Parkin (oxidized/aggregate preferring) | A15165D | Human | IHC-P, WB |
| Parkin (oxidized/aggregate preferring) | A15165E | Human | IHC-P |
| PINK1 | DU46-1.1 | Human | ICC, WB |
| Rab8 | A18073A | Human, Mouse, Rat | ICC, WB |
(Promotional pricing cannot be combined with other discounts. Price reduction will be reflected during checkout. If you are a customer who orders via a distributor, please contact your distributor for eligibility.)
Check out our other blog posts to learn more about Lewy body layers and the roles of Parkin.
Interested in receiving more related content? Sign up for our email updates.
References:
- Marotta et al. Organoid and pluripotent stem cells in Parkinson’s disease modeling: an expert view on their value to drug discovery. Expert Opin Drug Discov (2020). PMID: 31899983. DOI: 10.1080/17460441.2020.1703671
- Valadas et al. ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson's Disease. Neuron (2018). PMID: 29887339 DOI: 10.1016/j.neuron.2018.05.022
- Ludtmann et al. Monomeric alpha-synuclein exerts a physiological role on brain ATP synthase. J Neurosci (2016). PMID: 27733604. DOI: 10.1523/JNEUROSCI.1659-16.2016
- Ludtmann et al. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun (2018). DOI: 10.1038/s41467-018-04422-2
- Kim et al. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Reports (2019). PMID: 30799274. DOI: 10.1016/j.stemcr.2019.01.020
 Login / Register
Login / Register 






Follow Us