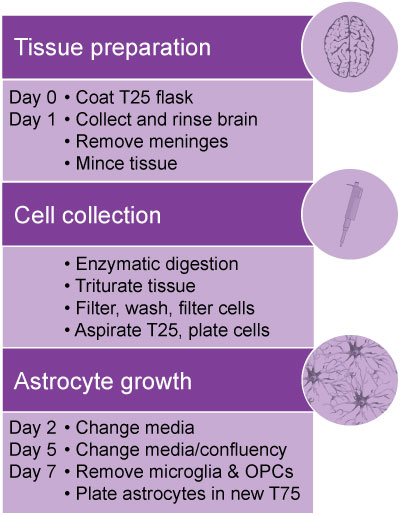
Isolation and Culture of Primary Mouse Astrocytes
Astrocytes are the major glial cells in the central nervous system and are involved in the regulation of numerous processes like energy metabolism, ion homeostasis, and synaptic plasticity. Primary astrocytes derived from rodent brains provide a rapid and easy solution for studying astrocyte biology and morphology. These cultured cells are an invaluable tool for elucidating underlying mechanisms of diseases such as:
This protocol demonstrates how to isolate and culture primary astrocytes from mouse pup brains. The purity and morphology of astrocytes is assessed by immunocytochemistry (ICC) using well-established astrocytic markers, including GFAP. |
 |
Reagent and Instrument List
|
|
Protocol
Note: Use aseptic techniques if you need to sort and grow cells in culture.
- Pre-warm 30 mL SFM and all ACM in water bath at 37°C.
- Harvest brains and transfer to a 35 mm tissue culture dish placed on ice.
- Gently rinse brains in cold SFM.
- Carefully aspirate the medium and transfer brains to a new 35 mm tissue culture dish containing cold SFM. Keep plate on ice.
- Remove the meninges using fine forceps to avoid contamination of the astrocyte culture with fibroblasts and meningeal cells. This step is performed under a dissecting (stereo) microscope.
- Transfer brain tissues to a new 35 mm dish containing 2 mL SFM.
- Cut brains into small pieces using a scalpel with sterile blade.
- Transfer brain pieces to a sterile 15 mL conical tube, and 5 mL of SFM and 1 ml 10x Trypsin/DNase solution to final total volume of 10 ml.
- Tighten the cap and gently mix by inverting the tube several times. Slightly loosen the cap and place the tube in the incubator (37°C, 5% CO2) for 30 min. Mix by occasional shaking every 10 min.
- Spin down at ~1200 RPM in a swinging-bucket centrifuge for 5 min.
- Carefully aspirate the supernatant and re-suspend the pellet with 10 mL ACM.
- To obtain a single cell suspension, dissociate the tissue by triturating with a 10 mL serological pipette 10 – 15 times. Avoid generating air bubbles. Let the suspension sit for 1 min.
- Place a 100 μm cell strainer on a 50 mL conical tube. Pass the top 5 mL of the cell suspension through the filter.
- Triturate the remaining suspension with a 5 mL serological pipette 10-15 times. Avoid generating air bubbles.
- Pass the remaining cell suspension through the strainer.
- Collect the filtered suspension and spin down at ~1200 rpm in a swinging-bucket centrifuge for 5 min.
- Re-suspend the pellet in 4 mL of ACM (use 1 mL of ACM per brain).
- Triturate with a 5 mL serological pipette 10-15 times.
- Place a 100 μm cell strainer on a 50 mL conical tube. Pass the cell suspension through the filter (a 40 μm cell strainer is preferred).
- Aspirate the coating medium (PLL or PDL) from the T25 tissue culture flask, which was pre-coated overnight.
- Collect and plate cells in a T25 tissue culture flask in total volume of 5 mL containing 2 mL of cell suspension and 3 mL of ACM.
- Leave the flask in the incubator (37°C, 5% CO2) overnight.
- Change media the next day, and then every 2-3 days. The astrocytes should grow confluent after 5-7 days.
- After 5-7 days, the microglial culture growing on the top layer may have detached from the astrocyte layer, and can be removed by gentle shaking (180 rpm) on an orbital shaker for 30 minutes, followed by aspiration and removal of the cell suspension. Fresh ACM can be added back to the flask.
- To dissociate and remove oligodendrocyte progenitor cells (OPCs), continue shaking the flask at 240 rpm for 5-6 hours. Remove the medium containing the detached OPCs.
- To expand the astrocyte culture, rinse cells with PBS 2x and then trypsinize with 5 mL of 0.25% tryspsin-EDTA until the cells are detached. Add 5 mL of ACM to the flask, collect and centrifuge the cell suspension at 1200 rpm for 5 min. Re-suspend cells in ACM and plate in two T75 flasks.
Representative Data
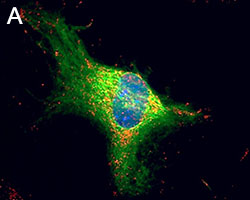
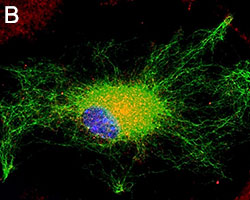
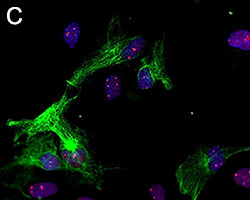
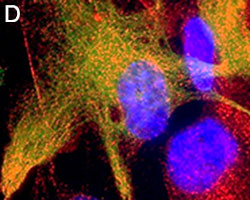
Primary astrocytes isolated from mouse brain tissue were cultured and stained for ICC. Cells were stained with anti-GFAP (clone SMI-25) Alexa Fluor® 488 (green), and either purified (A) anti-VDAC1 (clone N152B/23), (B) anti-GLUL (clone O91F4), (C) anti-MeCP2 (clone N227/21), or (D) ATF6 (clone W17028A) followed by an Alexa Fluor® 594 (red) secondary antibody (red). Nuclei were counterstained with DAPI and images were captured using a 60X objective.
 Login / Register
Login / Register 







Follow Us