MojoSort™ Mouse CD4+CD25+ Regulatory T Cell Isolation Kit
Reagent and Instrument List
|
Important Note
MojoSort™ magnetic particles can be used with other commercially available magnetic separators, both free standing magnets and column-based systems. Because MojoSort™ protocols are optimized for the MojoSort™ separator; the protocols may need to be adjusted for other systems. Please contact BioLegend Technical Service (tech@biolegend.com) for more information and guidance. We do not recommend using MojoSort™ particles for BD’s IMag™ or Life Technologies’ DynaMag™.
Protocol Steps
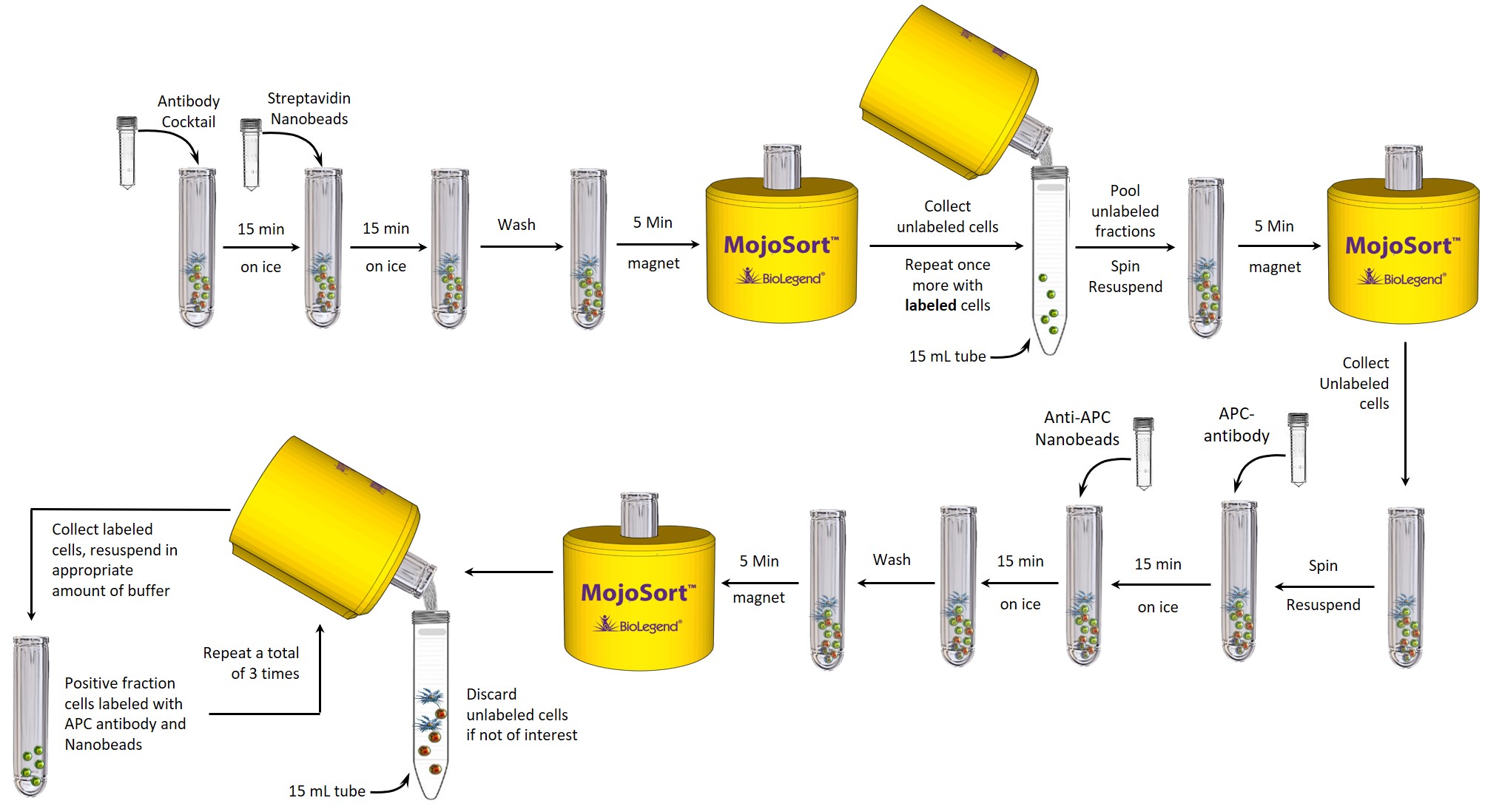
Product description and procedure summary:
Mouse CD4+CD25+ regulatory T cells are isolated using a combination of two cell separation steps. In the first step, non-CD4+ T cells are labeled with a biotin antibody cocktail and then incubated with magnetic Streptavidin Nanobeads (Cat. No. 480015/480016). While the unlabeled CD4+ T cells are poured to a clean tube, the magnetically labeled fraction is retained by the use of a magnetic separator. In the second step, the collected CD4+ T cells are labeled with an APC anti-mouse CD25 antibody and then incubated with anti-APC Nanobeads. The magnetically labeled CD4+CD25+ cells are retained by the use of a magnetic separator. Some of the downstream applications include functional assays, gene expression, phenotypic characterization, etc.
Note:
This procedure is optimized for the isolation of 108 cells per tube. For best results, optimize the conditions to your specific cell number and tissue. Prepare fresh MojoSort™ Buffer solution by diluting the 5X concentrate with sterile distilled water. Scale up volumes if using 14mL tubes and Magnet, and place the tube in the magnet for 10 minutes.
Sample Preparation:
- Prepare cells from your tissue of interest without lysing erythrocytes.
- In the final wash of your sample preparation, resuspend the cells in MojoSort™ Buffer by adding up to 4 mL in a 5 mL (12 x 75 mm) polypropylene tube.
Note: Keep MojoSort™ Buffer on ice throughout the procedure. - Filter the cells with a 70µm cell strainer, centrifuge at 300xg for 5 minutes, and resuspend in an appropriate volume of MojoSort™ Buffer. Count and adjust the cell concentration to 1 x 108 cells/mL.
Depletion of Non-CD4+ T Cells:
- Aliquot 1 mL of cell suspension (108 cells) into a 5 mL FACS tube.
Optional: Take an aliquot before adding the cocktail to monitor purity and yield. - Add 100 µL of the Biotin-Antibody Cocktail. Mix well and incubate on ice for 15 minutes.
Refer to the general guideline below when using less than 108 cells:
Starting Cell Number Amount of Biotin-Antibody Cocktail 1.0 x 107 cells
10 µL
5.0 x 107 cells
50 µL
- Resuspend the Streptavidin Nanobeads by vortexing at maximum speed, 5 touches.
- Add 100 µL of Streptavidin Nanobeads. Mix well and incubate on ice for 15 minutes.
Refer to the general guideline below when using less than 108 cells:
Starting Cell Number Amount of Streptavidin Nanobeads 1.0 x 107 cells
10 µL
5.0 x 107 cells
50 µL
- Wash the cells by adding MojoSort™ Buffer up to 4 mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard supernatant.
- Add 3.5 mL of MojoSort™ Buffer.
Note: If you observe aggregates, filter the suspension. To maximize yield, you can disrupt the aggregates by pipetting the solution up and down. - Place the tube in the magnet for 5 minutes.
Optional: Take a small aliquot before placing the tube in the magnet to monitor purity and yield. Keep unused cells to be used as control or other applications if needed. - Pour out the unlabeled fraction into a 15 mL conical tube. DO NOT DISCARD. Resuspend the labeled cells in 3.5 mL MojoSort™ Buffer.
- Repeat steps 11-12 on the labeled fraction once more for a total of 2 separations.
- Pool the unlabeled fractions and centrifuge at 300xg for 5 minutes.
- Discard the supernatant and transfer the cell pellet to a new 5 mL FACS tube. Resuspend in 3.5 mL MojoSort™ Buffer.
- Place tube in the magnet for 5 minutes to further increase the purity of CD4+ T cells.
- Pour out the unlabeled fraction into a new 5 mL FACS tube. This will be your enriched CD4+ T cells.
Positive Selection of CD25+ Cells:
- Centrifuge the unlabeled fraction at 300xg for 5 minutes. Discard the supernatant and resuspend the cell pellet in 100 µL MojoSort™ Buffer.
- Add 10 µL of the APC anti-mouse CD25 antibody. Mix well and incubate on ice for 15 minutes.
- Resuspend the Mouse anti-APC Nanobeads by vortexing at maximum speed, 5 touches.
- Add 10 µL of the Mouse anti-APC Nanobeads. Mix well and incubate on ice for 15 minutes.
- Wash the cells by adding MojoSort™ Buffer up to 4 mL. Centrifuge the cells at 300xg for 5 minutes.
- Discard supernatant.
- Add 2.5 mL of MojoSort™ Buffer.
Note: If you observe aggregates, filter the suspension. To maximize yield, you can disrupt the aggregates by pipetting the solution up and down. - Place the tube in the magnet for 5 minutes.
Optional: Take a small aliquot before placing the tube in the magnet to monitor purity and yield. Keep unused cells to be used as control or other applications if needed. - Pour out the unlabeled fraction into a 15 mL tube. Remove the tube from the magnet and resuspend the labeled cells in 2.5 mL MojoSort™ Buffer, these are the CD4+CD25+ T Cells.
- Repeat steps 25-26 on the labeled fraction twice more for a total of 3 separations.
- The APC labeled CD4+CD25+ regulatory T cells are now ready for use.
Chart Protocol
 Login / Register
Login / Register 







Follow Us