- Regulatory Status
- RUO
- Other Names
- Control cells
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

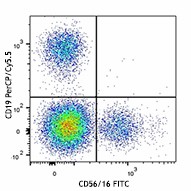
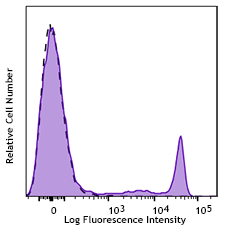
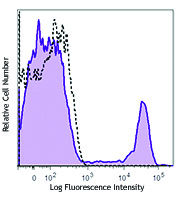
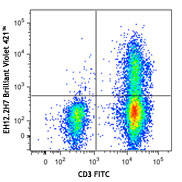
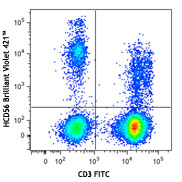
Veri-Cells™ PBMC were stained with anti-human CD19 (clone HIB19) PerCP/Cy5.5, CD16 (clone 3G8) FITC, and CD56 (clone HCD56) FITC (top) or CD4 Brilliant Violet™ 510 and FOXP3 (clone 206D) PE (bottom). -

The reconstituted lyophilized human PBMC can be used for immunofluorescent staining and multi-color flow cytometry assays. The cells can be used as controls for stability studies to avoid donor variability. These cells have been verified to work with commonly tested cell markers such as CD3, CD4, CD8, CD19, CD20, CD56/16, CD14, CD25, CD69, Granzyme B, Perforin, FOXP3, and Helios. View all 150 markers verified by LEGENDScreen™.
This product contains human cells, a potentially biohazardous material. Blood used in preparation of these samples was tested and found to be negative, using FDA approved methods, for antibody and nucleic acid testing against Human Immunodeficiency virus (HIV), Hepatitis B and C virus and HTLV (Human T-Lymphotrophic virus I and II), surface antigen for Hepatitis B (HBsAg) virus and syphilis and West Nile Virus. Biological tests are not 100% accurate. Use standard precautions when handling and treat as if the product is capable of transmitting disease. When handling or disposing, follow precautions described in CDC and FDA recommendations and OSHA Bloodborne Pathogen guidelines.
Product DetailsKit Contents
- Kit Contents
-
Cat. No. 425001
- 1 vial of Veri-Cells™ PBMC (> 5 x 106 cells)
- 1 vial of 1.5 ml Veri-Cells™ Buffer A Plus
Cat. No. 425002
- 4 vials of Veri-Cells™ PBMC (> 5 x 106 cells per vial)
- 4 vials of 1.5 ml Veri-Cells™ Buffer A Plus each
Cat. No. 425003
- 2 vials of Veri-Cells™ PBMC (> 1 x 106 cells per vial)
- 1 vial of 700 µl Veri-Cells™ Buffer A Plus each
Cat. No. 425004
- 10 vials of Veri-Cells™ PBMC (> 1 x 106 cells per vial)
- 5 vials of 700 µl Veri-Cells™ Buffer A Plus each
Veri-Cells™ Buffer A Plus is an improved version of the original Veri-Cells™ Buffer A to improve staining performance with tandem dyes.
Product Details
- Formulation
-
Cat. Nos. 425003 and 425004: ≥ 1 X 106 lyophilized human peripheral blood mononuclear cells (PBMC).
Cat. Nos. 425001 and 425002: ≥ 5 X 106 lyophilized human peripheral blood mononuclear cells (PBMC). - Preparation
- The Veri-Cells™ PBMC Kit is prepared from lyophilized human PBMC and a vial of Veri-Cells™ Buffer A Plus.
- Storage & Handling
- Store the kit at 2°C - 8°C upon receipt. Do not open sealed pouch until ready to use. Once reconstituted, the cells may be used for up to five days if properly stored at 2°C - 8°C in the buffer provided. Contact BioLegend Technical Support for information on the current lots.
- Application
-
FC - Quality tested
ICFC - Verified - Recommended Usage
-
Cat. Nos. 425001, 425002
50 µl per test (after reconstitution with 1.3 ml)
Cat. Nos. 425003, 425004
50 µl per test (after reconstitution with 325 µl)
Once reconstituted, let the cells sit for 15 minutes at room temperature prior to staining.
Veri-Cells™ Buffer A Plus may show precipitation over time, however this is normal and does not affect performance of the buffer. - Application Notes
-
The Veri-Cells™ PBMC Kit can be used as positive controls for surface immunofluorescent staining and multi-color flow cytometry assays using the BioLegend surface staining protocol. It can also be used for the detection of intracellular molecules such as Granzyme B, Perforin, Foxp3 and Helios using BioLegend’s Fixation Buffer and Intracellular Staining Permeabilization Wash Buffer (10X) or the True Nuclear™ Transcription Factor Buffer Set. For best results, Cell Staining Buffer is recommended for any washing steps. Product performance specifications are based on testing with BioLegend reagents and protocols. Deviations from the use of these reagents and procedures have not been assessed and may affect performance.z
Cell subset
Acceptance Criteria, % of lymphocyte gate
CD3+
55-95
CD4+CD3+
30-65
CD8+CD3+
10-55
CD19+CD3 –
3-40
CD56+CD16+CD3 –
3-40
Note on indicated ranges: Percent positive values are obtained based on gates set on unstained cells. The antibodies used for testing were the following: CD56 PE (clone 5.1H11), CD16 PE (clone 3G8), CD19 APC (clone HIB19), CD3 FITC (clone UCHT1), CD4 PE/Cy7 (clone RPA-T4), and CD8 APC/Cy7 (clone RPA-T8). Variability in antibodies used, instruments, staining conditions and other factors may influence end-user values. The Certificate of Analysis specific values are provided as a reference. It is recommended that individual laboratories establish its own control values and ranges for each parameter based on laboratory specific conditions and protocols.
Please use our CoA Look-Up Tool to find the expected lot-specific frequencies of certain cell populations included within each lot of Veri-Cells™ PBMC. The antibodies used for testing were the following: CD56 PE (clone 5.1H11), CD16 PE (clone 3G8), CD19 APC (clone HIB19), CD3 FITC (clone UCHT1), CD4 PE/Cy7 (clone RPA-T4), CD8 APC/Cy7 (clone RPA-T8). Percent positive is based on gates set on unstained cells. The Certificate of Analysis specific values are provided only as a reference. Variability in antibodies used, instruments, staining conditions and other factors may influence end-user values. It is recommended that individual laboratories establish its own control values and ranges for each parameter based on laboratory specific conditions and protocols. - Additional Product Notes
-
View more applications data using this product to characterize their use as a control in flow cytometry assays and longitudinal studies in our Scientific Poster Library.
Veri-Cells™ Buffer A Plus is an improved version of the original Veri-Cells™ Buffer A to improve staining performance with tandem dyes. - Product Citations
-
Antigen Details
- Biology Area
- Immunology
- Molecular Family
- CD Molecules
- Gene ID
- NA
Related Pages & Pathways
Pages
Related FAQs
- Is washing required after staining?
-
Yes, it is recommended to wash after staining. We recommend using BioLegend's Cell Staining buffer (Cat. No. 420201) for the wash step.
- Can the cells be activated after rehydration?
- No, they cannot be activated.
- Are these cells virus/pathogen free?
- These are tested to be free of HIV, HBV, syphilis, and HCV.
- Are there any RBCs in Veri-cells™?
- There may be a few, but the majority of RBCs are not present in Veri-Cells™ preparations.
- Are these cells viable?
- No they are not.
- Can a constant percentage of each population be expected in each lot?
- Yes, within each lot, cell populations remain constant.
- Are the cells fixed?
-
They are treated with our proprietary mixture prior to lyophilization.
- Which markers can be stained?
-
160+ known markers have been verified - please view markers verified by LEGENDScreen™ or contact tech@biolegend.com for a complete list.
- Can they be used to detect intracellular cytokines such as TNF-α or IFN-γ?
-
For this, use Veri-Cells™ Activated (Cytokine) PBMC which have been activated with Cell Activation Cocktail (with Brefeldin A) containing PMA and Ionomycin. These have been validated to stain positive for activation-induced cytokines including TNF-α and IFN-γ. Veri-Cells™ PBMC and Veri-Cells™ CD4-Low PBMC will not have detectable TNF-α or IFN-γ.
- How do we QC these cells?
-
Each Veri-Cells™ product is tested with a panel of antibodies to ensure expression of these markers falls within the expected range. For more information regarding the exact markers used for each product, please contact technical service.
 Login / Register
Login / Register 













Follow Us