A Sparkling Advancement for Flow Cytometry
|
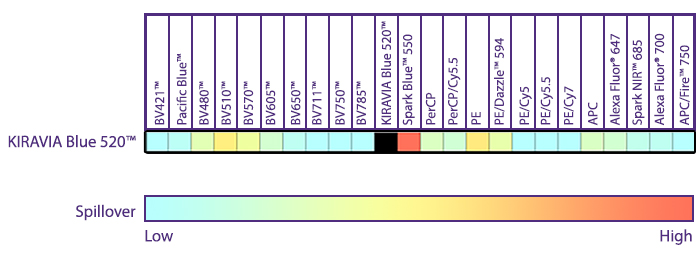
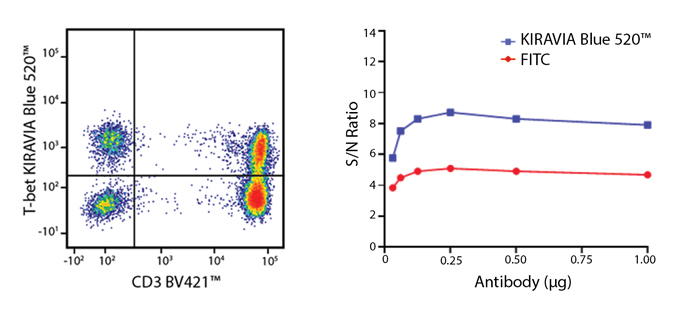
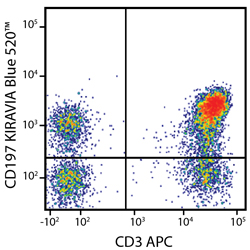
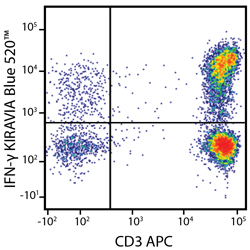
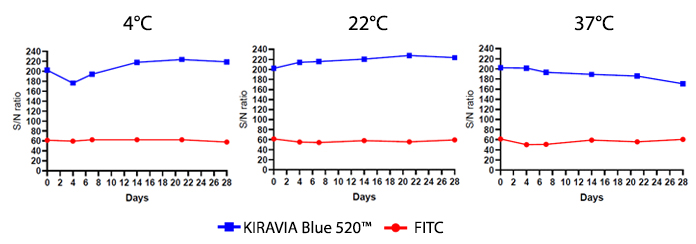
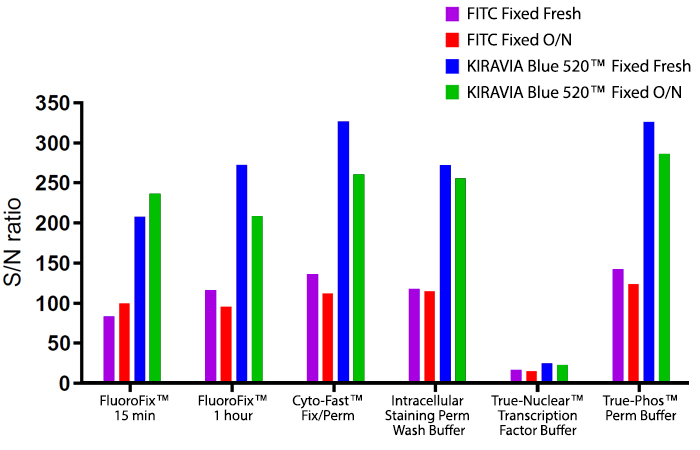
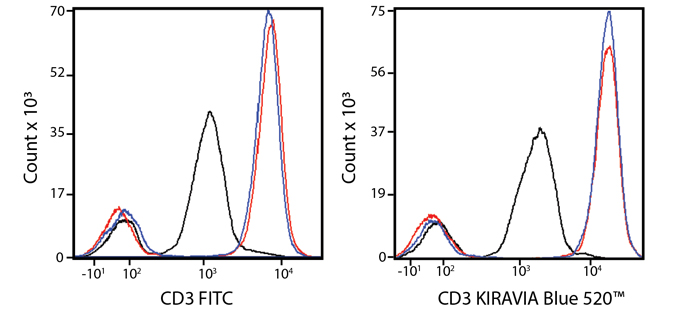
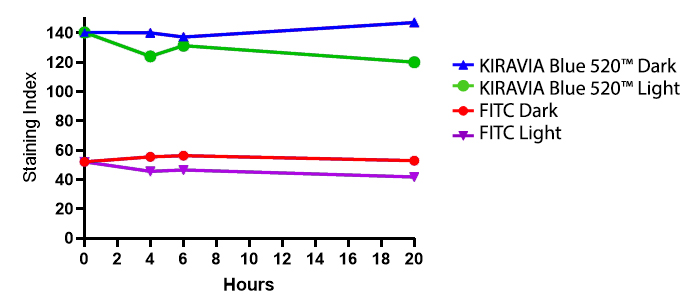
Introducing the KIRAVIA Dyes™, a brand new fluorescent chemistry to advance applications in multicolor flow cytometry and beyond. KIRAVIA is a coined term meaning "sparkling" in Japanese. KIRAVIA Blue 520™ is the first in a new family of fluorophores. This class of dyes employs a unique organic backbone that separates fluorophores to minimize quenching effects, thus allowing optimal and higher fluorophore to protein (F:P) ratios, surpassing what is possible with direct conjugations of single fluorophores like FITC.
View our full listing of KIRAVIA Blue 520™ antibodies and contact us to try a sample today.
KIRAVIA Dyes™ provided by Sony.
|
|
KIRAVIA Blue 520™ Spectra
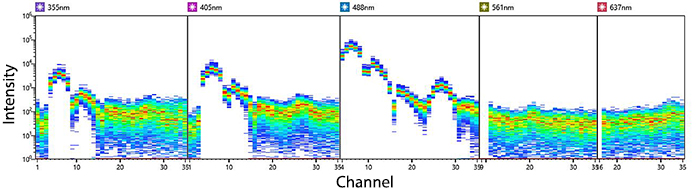
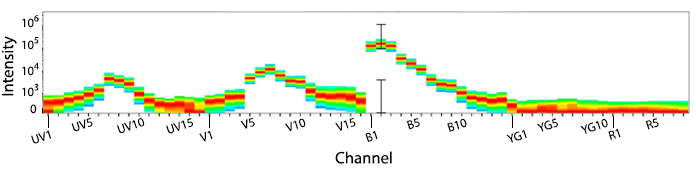
Emission spectra of KIRAVIA Blue 520™ as run on a (top) SONY ID7000™ Spectral Cell Analyzer or (bottom) 5-laser Cytek™ Aurora Spectral Cytometer.
 Login / Register
Login / Register 







Follow Us