Uncover the Astrocyte Profile in Alzheimer’s Disease

Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that impairs cognitive function and interferes with the ability to carry out normal daily tasks. The two most common pathological hallmarks of AD are the generation of toxic amyloid beta (Aβ) peptides and their accumulation in amyloid plaques, and hyperphosphorylation of Tau protein leading to the formation of Tau tangles. These events compromise neuronal cell health and ultimately lead to cell death. While tremendous progress has been made in understandisung the underlying molecular mechanisms leading to neuronal loss, it is unclear how non-neuronal cells, such as astrocytes, may contribute to disease pathology or progression. In recognition of Alzheimer’s Awareness Month, we are highlighting a recent study that characterizes the cellular landscape of AD by defining the genome and proteome of disease-associated astrocytes.
The Role of Astrocytes
Astrocytes are the most abundant glial cell type in the brain, accounting for 20 to 40% of all glial cells, and can be identified with markers such as GFAP and S100β. Astrocytes perform many functions, such as providing metabolic support for neurons and helping to maintain blood-brain barrier (BBB) integrity. Astrocytes are highly sensitive to alterations in their microenvironment. For example, in response to local injury, they undergo morphological changes and alter their gene expression profile to upregulate expression and secretion of a variety of bioactive molecules, such as cytokines and chemokines.
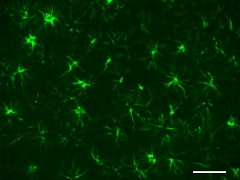
Formalin-fixed, paraffin-embedded rat brain tissue stained with anti-GFAP (clone SMI 24) Alexa Fluor® 488.

Sequencing Alzheimer’s Disease-Associated Astrocytes
In recent years, novel microglial cellular populations associated with disease stages have been identified using single-cell technologies(1). However, a similar disease-related astrocytic cellular diversity has not yet been fully explored. To gain a better understanding of cellular and functional diversity of astrocytes in relation to AD, Habib et al.(2) used single-nucleus RNA sequencing (sNuc-seq) to identify transcriptional states of AD-associated astrocytes in an AD transgenic mouse model (5xFAD). Using this approach, they discovered a unique transcriptional state that was only observed in AD, but not in wild type (WT) mice. Cells with this unique profile were termed disease-associated astrocytes (DAAs). DAAs were detected in male and female mice and had high GFAP expression. DAAs had upregulated genes that were associated with inflammation, toxic response, and metabolic pathways. Notably, DAAs upregulated Serpina3n and Ctsb, which produce proteins linked to Aβ accumulation and the processing of amyloid precursor protein, respectively.
Spatial Positioning and Protein Expression of DAAs
The group also characterized the spatial distribution of DAAs by immunohistochemistry using antibodies for protein detection: GFAP, a marker of reactive astrocytes; vimentin, a marker for adult neurogenesis; and Serpin A3N, the aforementioned serine protease inhibitor that can affect Aβ accumulation. Triple positive astrocytes were only detected in AD mice and abundant in the subiculum, where large Aβ deposits and gliosis are observed(3). In addition, GFAP and vimentin positive astrocytes were abundant in the hippocampus and the subiculum. Serpin A3N and vimentin positive astrocytes were also found in close proximity to amyloid plaques in these mice. In healthy WT mice, vimentin-positive astrocytes were found in the dentate gyrus of the hippocampal formation, which is consistent with this neurogenic niche of the brain. These observations are aligned with previous findings describing amyloid plaque-mediated activation of astrocytes(4, 5).
BioLegend Reagents for Alzheimer’s Research
Research by Habib et al. demonstrates how different applications can be combined to validate novel findings. BioLegend offers a wide range of solutions to profile various cell types and to detect AD-related pathology. Our gold-standard antibodies can be used to distinguish astrocytes from other cells types in the brain, and they can identify the pathological hallmarks of AD using specific antibodies for Aβ peptide and Tau protein. Explore a selection of our Alzheimer’s disease products in the table below.
For proteogenomics analysis, our TotalSeq™ oligo-conjugated antibodies allow simultaneous high-parameter protein and RNA profiling at a single-cell level. For additional support, you can find research tools on our Neuroscience Webpage or reach out to us to discuss collaborations and custom reagents.
Interested in receiving more related content? Sign up for our email updates.
Request a copy of our 2021 From Research to Cure Wall Calendar.
| Featured Antibodies for Alzheimer's Research | |||
|---|---|---|---|
| Specificity | Clone | Reactivity | Application |
| APP, Aβ peptides | 6E10 | Human | IHC-P, WB, ELISA |
| APP, Aβ peptides | M3.2 | Mouse, Rat | WB, ELISA |
| Aβ 1-42 | 12F4 | Human, Mouse, Rat | IHC-P, WB, ELISA |
| Aβ 1-40 | 11A50-B10 | Human, Mouse, Rat | IHC-P, WB |
| α-Synuclein, aggregated | A17183A | Human | IHC-P |
| Aggregated Aβ | A17171C | Human | IHC-P, WB |
| Aggregated Aβ | 3A1 | ||
| APP/Aβ Antibody Sampler Kit | 6E10, 12F4, 3A1, M3.2, 11A50-B10 | Human, Mouse, Rat | IHC-P, WB, ELISA |
| Tau Phospho (Ser262) | A15091A | Human | IHC-P, WB, ELISA |
| Tau, 1-223 | A16103A | Human | IHC-P, WB, ELISA |
| Tau, 368-441 | A16097F | Human | IHC-P, WB, ELISA |
| Tau Phospho (Thr181) | M7004D06 | Human | IHC-P, WB, ELISA |
| Tau Antibody Sampler Kit | A15091A, A16103A, A16097F, M7004D06 | Human | IHC-P, WB, ELISA |
| GFAP | SMI 24 | Human, Mouse, Rat | IHC-P, WB |
| GFAP | SMI 25 | Human, Mouse, Rat | IHC-P, WB |
| ALDH1L1 | N103/39 | Human, Mouse | WB |
| Glutamine Synthetase | O91F4 | Human, Mouse, Rat | IHC-P, ICC, WB |
| Glial Cell Marker Antibody Sampler Kit | 8E10.D9, S1600D, SMI 24, SMI 91, P82H9 | Human, Mouse, Rat | IHC-P, WB |
References:
- Keren-Shaul et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 2020; 169 (7): P1276-1290.
- Habib et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nature Neuroscience. 2020; 23: 701-706.
- Oakley et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer's Disease Mutations: Potential Factors in Amyloid Plaque Formation. J Neurosci. 2006; 26 (40): 10129-10140.
- Matias et al. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front Aging Neurosci. 2019; 11; 59.
- Rodriguez-Arellano et al. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016; 323; 170-182.
 Login / Register
Login / Register 






Follow Us