The Rat Effectively Models Signature Cytokines of T Helper Cells
The vertebrate immune system has multiple lines of defense against potentially dangerous pathogens or toxins. In this blog, we review the mammalian immune system, the continuing value the rat animal model brings to physiological research, and the importance of sensitive immunoassays that measure signal molecules central to immune responses.
Innate immunity is our first line of defense and consists of barriers such as skin, saliva, and a number of other non-specific immune responses that serves to eliminate any substance that is ‘non-self’. A dangerous substance that gets past innate immunity then faces adaptive immunity (also known as acquired immunity). Our adaptive immunity is characterized by T and B cells, a portion of which gives rise to long-lived memory cells that remain in circulation and form the basis of immunological memory. Upon activation, there are two classes of T cells: CD8+ cytotoxic T cells and CD4+ T helper (Th) cells. Cytotoxic T cells can destroy infected cells, whereas Th cells activate macrophages, B cells, and cytotoxic T cells. Upon additional signals, Th can differentiate into effector Th cells such as Th1, Th2, Th9, Th17, and Th22, each of which secretes a subset of cytokines that contributes to modulation of other immune cells and elimination of pathogens or parasites.
Given the complexity of mammalian physiology, it’s not surprising that animal models, such as rodents and pig, have contributed a great deal to our knowledge of human immune responses and other complex physiological systems. While mice strains have been the to-go model for mammalian genetics research, the rat is considered a choice model to study more complex physiological processes for a variety of reasons. For example, the rat’s larger size allows for serial blood draws, the collection of larger cell numbers, and more sophisticated surgeries1. Such manipulations are nearly impossible using mice. The rat serves as the gold standard for disease and transplant studies because it models the immunobiology and immunopathology of many human organs2. The rat is heavily utilized for neuroscience3, and the rat shares important immune characteristics with humans that are missing in mice4.
BioLegend understands that immunology research advances with the help of animal models, and so we offer LEGENDplex™ immunoassays with reactivities in human, mouse, rat, and non-human primates. These assays utilize multiple beads, with each bead conjugated with an immobilized antibody on its surface that serves to capture a target cytokine analyte. Each population of beads is distinguished by size and internal fluorescence, allowing for the simultaneous analysis of multiple cytokines in a single sample, thus saving on workflow and samples. To capitalize on the rat model, we now offer LEGENDplex™ Rat Th Cytokine Panel (13-plex) V02. This expands our line from LEGENDplex™ Human Th Cytokine Panel and LEGENDplex™ Mouse Th Cytokine Panel, both of which measure 12 cytokines: IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-17A, IL-17F, IL-22, IFN-γ and TNF-α, subsets of which are secreted by effector Th1, Th2, Th9, Th17, or Th22 classes.
As the newest member of this ensemble, the LEGENDplex™ Rat Th Cytokine Panel (13-plex) V02 measures the abovementioned 12 cytokines along with granulocyte-macrophage colony-stimulating factor (GM-CSF). This cytokine promotes the proliferation of bone marrow-derived macrophages and granulocytes, but an imbalance in its production or signaling has been implicated in autoimmune diseases5. In addition, as our representative data highlights, our Rat Th Cytokine Panel V02 supports samples from a variety of organs, including brain, lung, and spleen, to name a few. Don’t need the entire panel? No problem, we also offer subpanels that focus on cytokines secreted by Th1, Th2, Th17, Th1/Th2, or Tfh, thus providing researchers utilizing the rat model an expansive toolkit to pursue their immunology research goals.
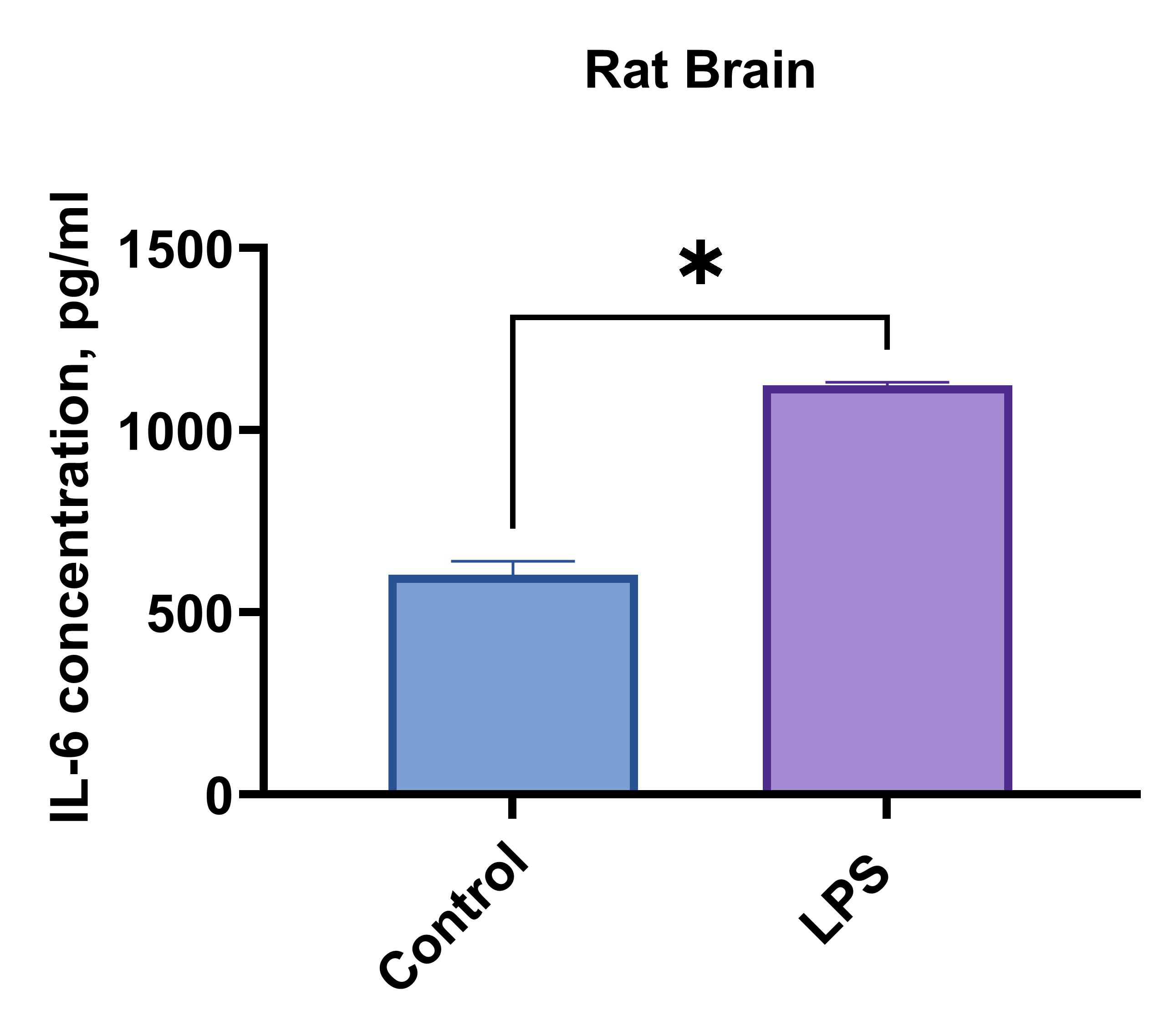
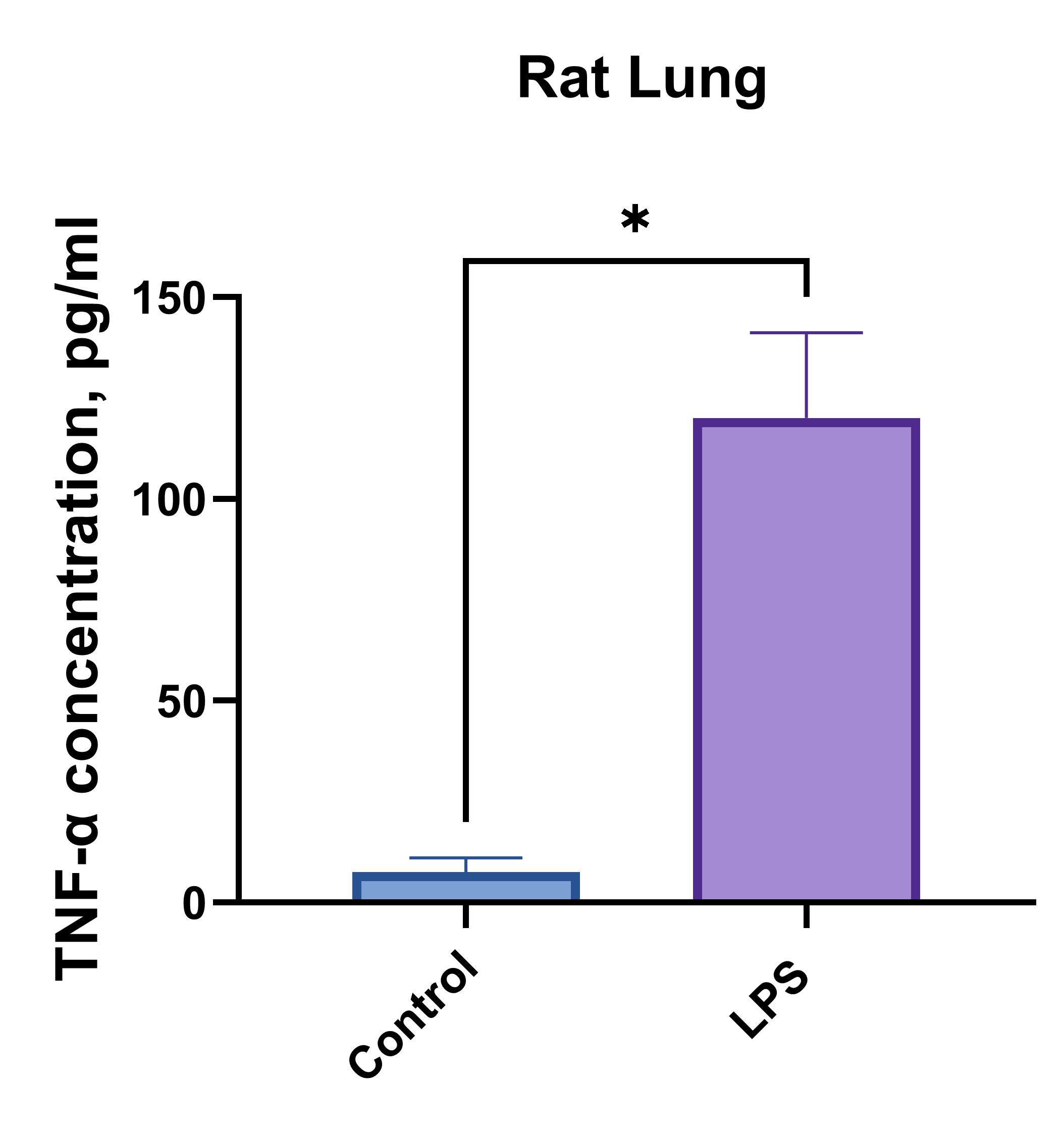
Rat brain or lung cells (1 x 106 cells/mL) were cultured under LPS (1 µg/mL) stimulation or no treatment (Control). Supernatants were collected after 96 hours and assayed with the LEGENDplex™ Rat Th Cytokine Panel (13-plex) V02.
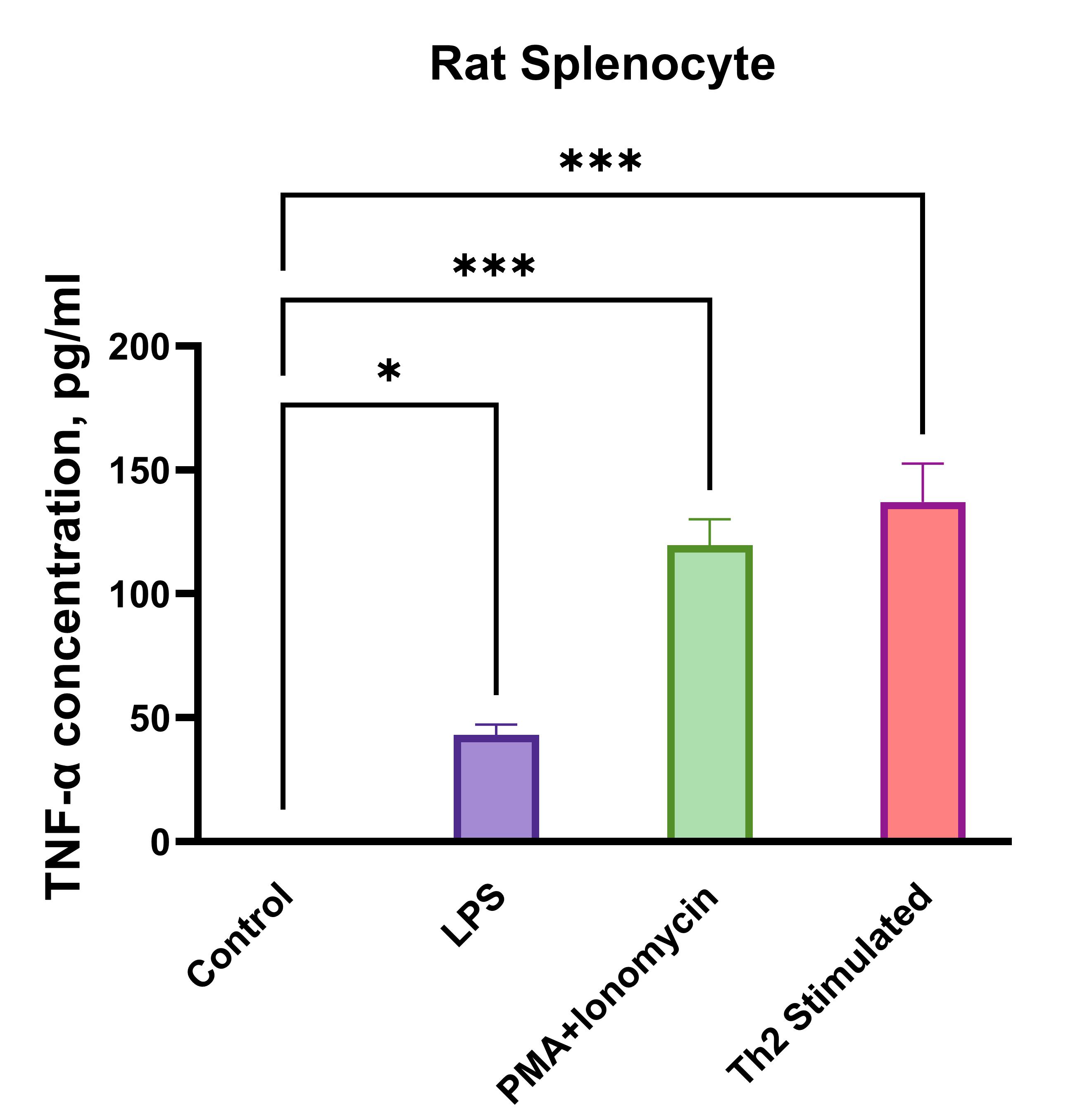
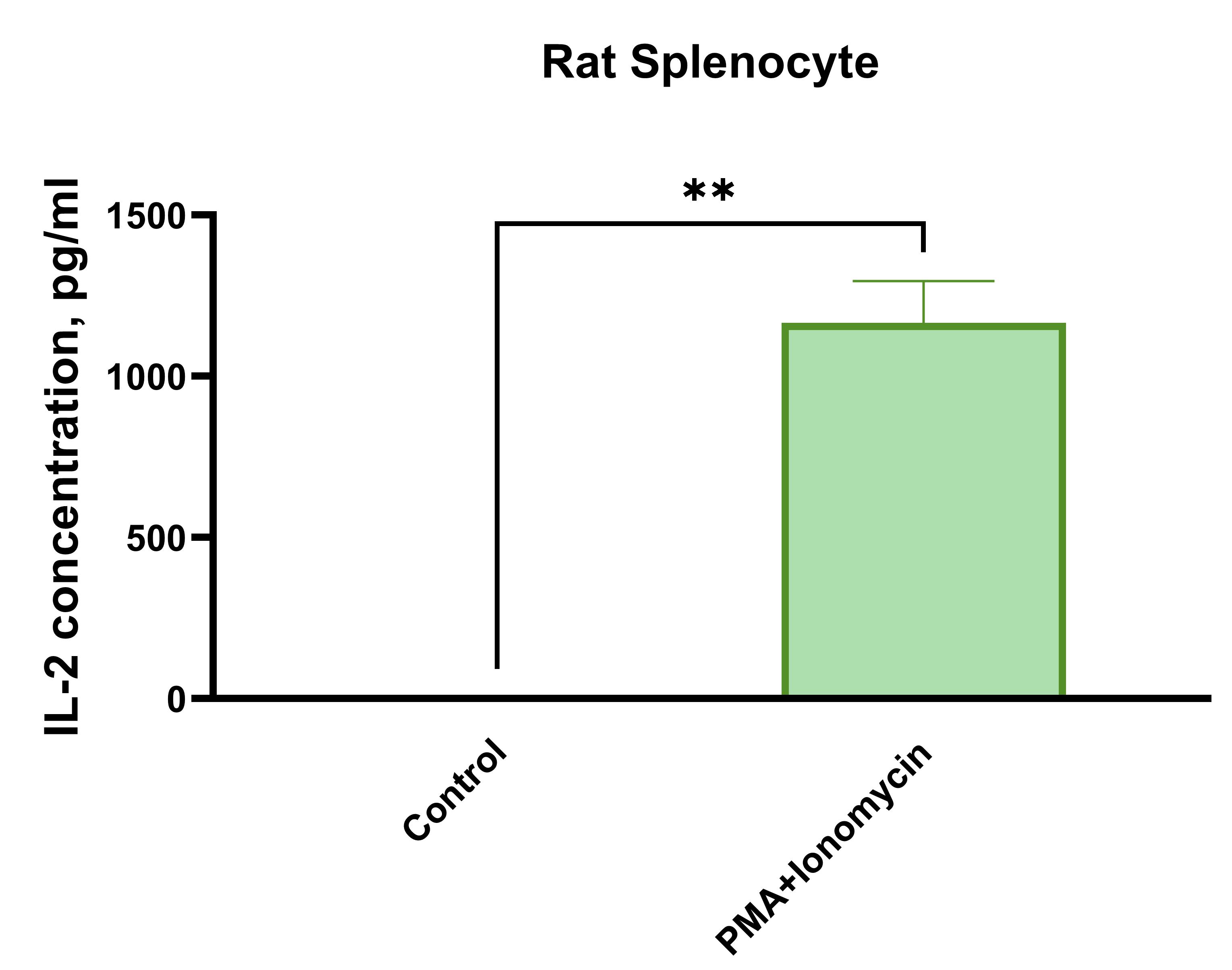
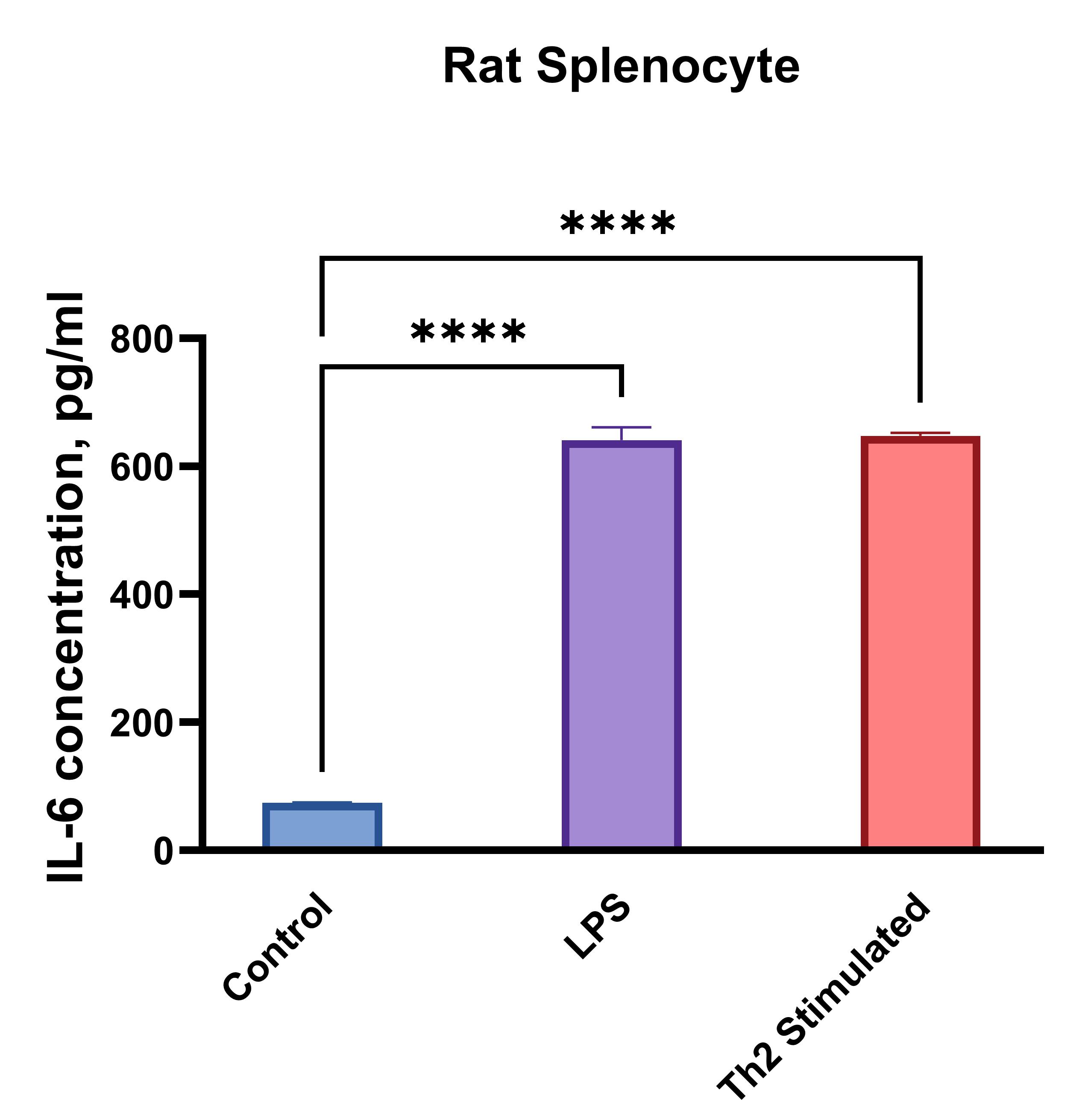
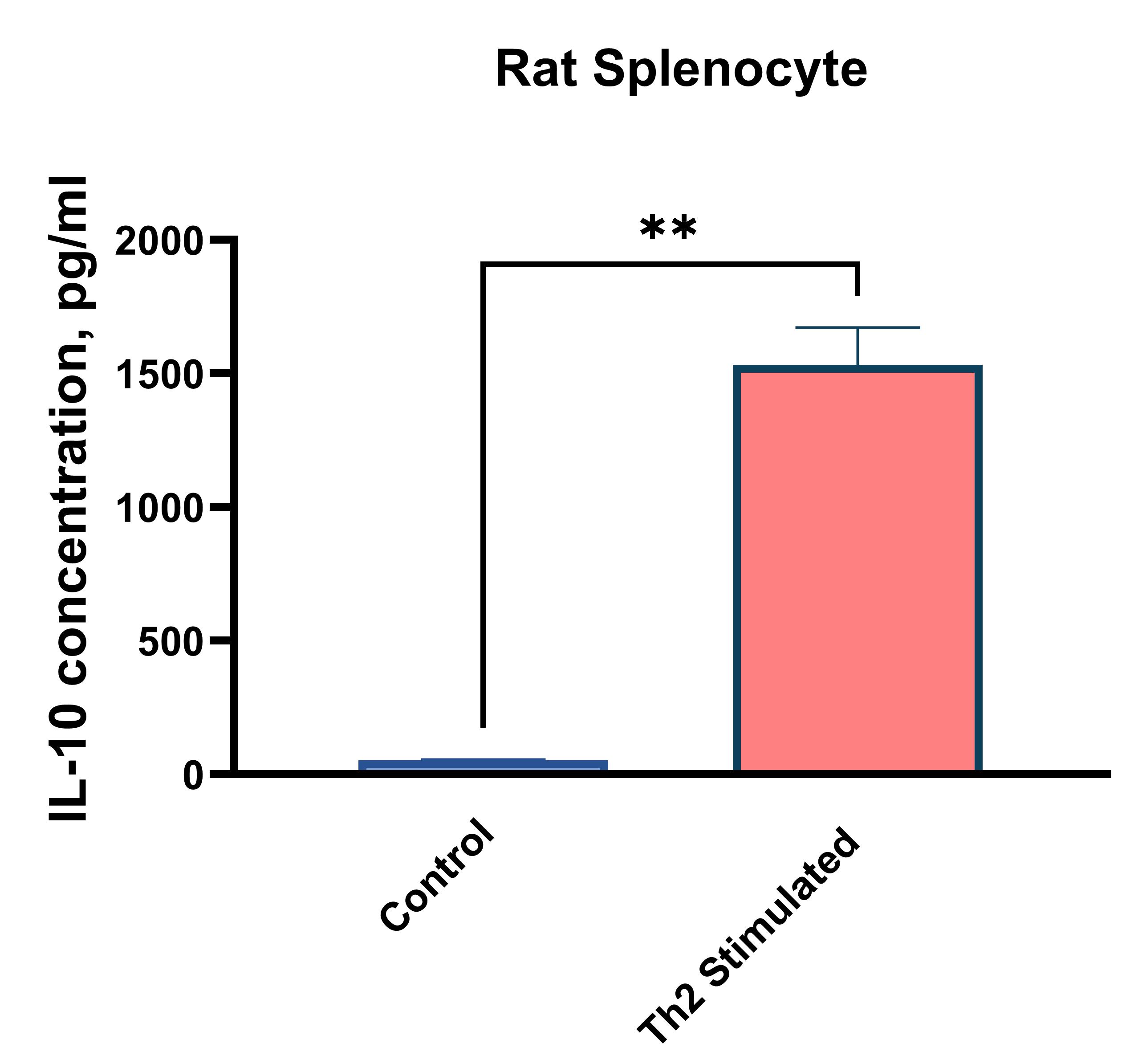
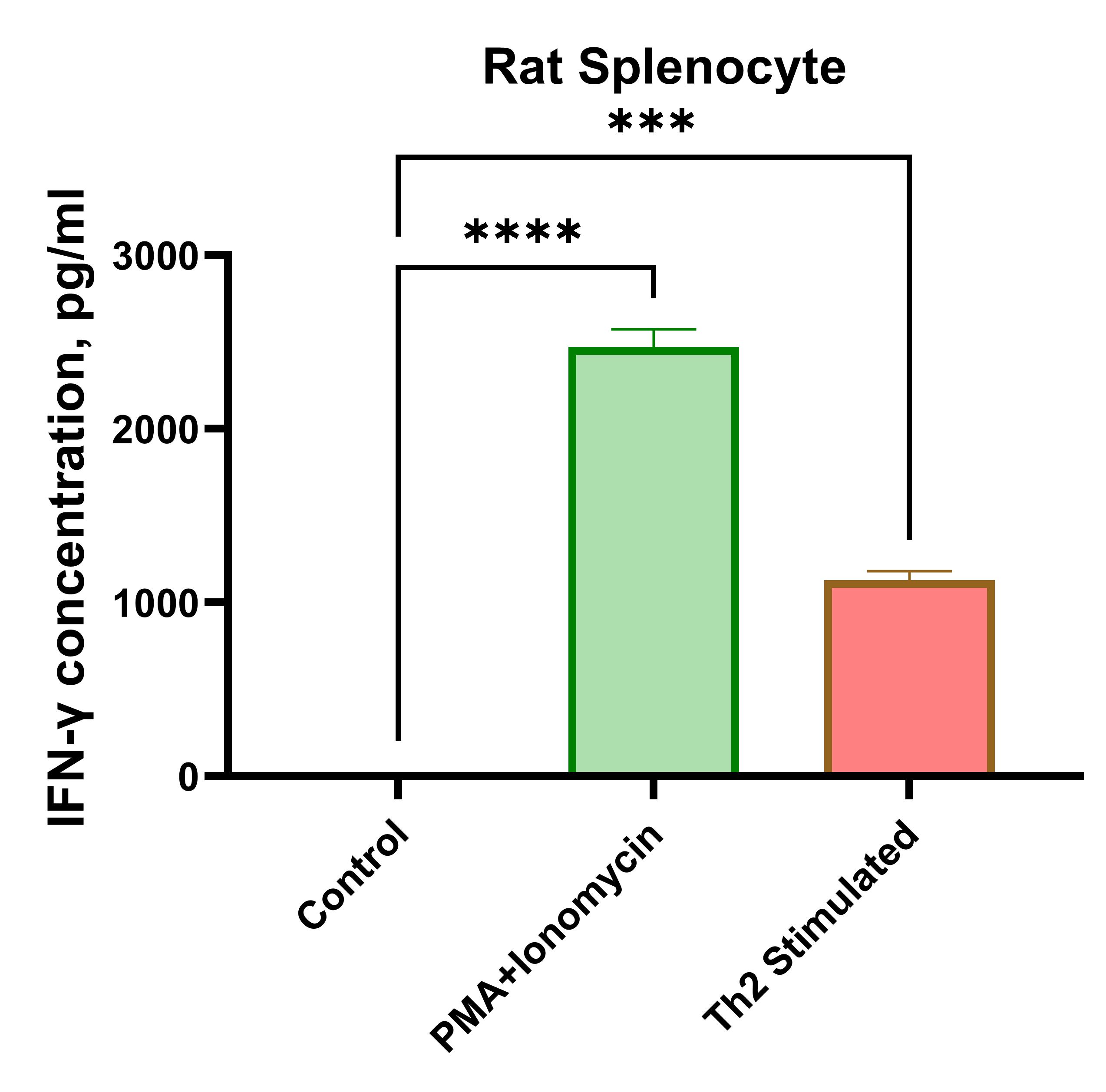
Rat splenocytes (1 x 106 cells/mL) were cultured under the following treatments: Control (no treatment), LPS (1 µg/mL), Th2 stimulation*, or PMA (20 ng/mL) + Ionomycin (500 ng/mL). Supernatants were collected after 96 hours and assayed with the LEGENDplex™ Rat Th Cytokine Panel (13-plex) V02. * Th2 polarization was performed by plate bound anti-CD3 (1 ug/mL) + soluble anti-CD28 (1 ug/mL) + anti-IFN-y (10 ug/mL) + IL-4 (40 ng/mL) + IL-2 (10 ng/mL).
Cytokines secreted by effector Th cells mediate activities of other effector immune cells but are also involved in inflammatory and autoimmune diseases. In this blog, we emphasize that accurate measurement of Th cytokine expression is critical for in-depth research of immune responses and diseases. Biomedical research will only progress with innovative tools and approaches, such as the use of rat and other animal models. BioLegend recognizes the contributions to human health brought by animal models, and we’re proud to offer an array of assays to support your multi-parameter analysis of the immune and other physiological systems.
References
- Szpirer, Claude. “Rat models of human diseases and related phenotypes: a systematic inventory of the causative genes.” Journal of biomedical science vol. 27,1 84. 2 Aug. 2020, doi:10.1186/s12929-020-00673-8 PubMed
- Wang, Xinle et al. “Immunological Determinants of Liver Transplant Outcomes Uncovered by the Rat Model.” Transplantation vol. 105,9 (2021): 1944-1956. doi:10.1097/TP.0000000000003598 PubMed
- Sahu, Upasana et al. “Rat and Mouse Brain Tumor Models for Experimental Neuro-Oncology Research.” Journal of neuropathology and experimental neurology vol. 81,5 (2022): 312-329. doi:10.1093/jnen/nlac021 PubMed
- Ménoret, Séverine et al. “In Vivo Analysis of Human Immune Responses in Immunodeficient Rats.” Transplantation vol. 104,4 (2020): 715-723. doi:10.1097/TP.0000000000003047 PubMed
- Lotfi, Noushin et al. “Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update.” Frontiers in immunology vol. 10 1265. 4 Jun. 2019, doi:10.3389/fimmu.2019.01265 PubMed
 Login / Register
Login / Register 






Follow Us