Microglia: The Immune Cells of the Brain
| In one of my previous blogs, I discussed neurons and their role in the function of the brain. In today’s blog, I will talk about glial cells. More specifically, about microglia and their role in innate immunity in the brain. Although glial cells were first identified in 1824, they were always overshadowed by their more popular partner cells, the neurons. Interest in glial cells picked up with the invention of the electron microscope post-1950, when several scientists started investigating the ultra-structure of these cells. Previously it was thought that glial cells were outnumbered by neurons 10:1. However, new data estimates that glial cells are present in equal proportion to neurons, although the ratio differs from one part of the brain to another. |
|
As the Greek name suggests (glia means glue), glial cells are commonly known as the glue of the nervous system that surround the neurons and hold them in place. Other functions of glial cells include supplying nutrients and oxygen to neurons, destroying pathogens and removing dead neurons, and insulating neurons from one another. For a long time it was believed that glial cells did not play a role in neurotransmission, however, glial cells have recently been found to assist neurons in forming synaptic connections (1) and in other processes, such as synaptic plasticity. |
|
| There are two main types of glial cells: microglia and macroglia. The central nervous system (CNS) consists of different types of macroglial cells including astrocytes and oligodendrocytes. The peripheral nervous system (PNS) contains other types of glial cells like the Schwann cells. All these different macroglial cell types have been found to have specific functions. For example, astrocytes are the main building block of the blood-brain-barrier (BBB), and oligodendrocytes coat the axons in the CNS forming the myelin sheath. So, as you can see, these cells are no less important than the neurons! Considered as tissue-resident macrophages, microglia are the only cells in the CNS that are of hematopoietic origin (2). Although microglia are important in maintaining normal homeostasis in non-infected regions, these cells are also constantly patrolling the CNS and scavenging for foreign materials, plaques, and damaged neurons. A crucial function of microglia is the ability to generate significant innate and adaptive immune responses. When infectious agents cross the BBB, or when there is an injury, microglia are the first responders to decrease inflammation and destroy the infectious agents by phagocytosis before they can damage the neural tissue. Microglia express numerous Toll-like receptors (TLR), a major family of pattern recognition receptors (PRRs) that are critical for recognizing conserved motifs expressed by a wide array of pathogens (3). Further, microglia can rapidly upregulate expression of MHC class I and II proteins and become efficient antigen presenting cells to T cells that have crossed the BBB (4). |
|
| Activation of microglia in response to inflammation is a hallmark of brain pathology. In their native resting state, microglia have a ramified structure comprised of numerous branching processes and contain little cytoplasm. These extended processes allow microglia to rapidly sense the presence of tissue damage or signs of infection through PRRs. Following activation, these cells change their morphology and become more rounded. In the activated form, microglia are traditionally divided into two states: M1 (classical), and M2 (alternative), depending on the nature of the stimulus (5). In the M1 proinflammatory state, activated microglia produce oxidative metabolites and proinflammatory cytokines (such as TNF-α and IFN-γ) that induce helper T cell responses, and assist in killing of intracellular pathogens. In contrast, M2 is an anti-inflammatory state that promotes tissue repair, matrix remodeling and neuroprotection through secretion of anti-inflammatory cytokines (such as TGF-β and IGF-1). | |
| Besides secreting cytokines, microglia are also sources of certain chemokines such as MCP-1, MIP-1α and MIP-1β, that are capable of recruiting T cells to the site of inflammation. Microglia can also synthesize and secrete proteolytic enzymes such as cathepsins and matrix metalloproteinases (MMP) that can degrade both the extracellular matrix and neuronal cells. | 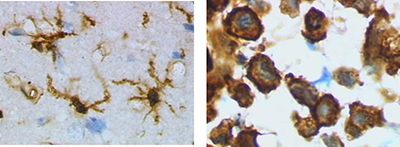 Left Image: Ramified form of microglia from rat cortex before traumatic brain injury (lectin staining with HRP). Right Image: Activated form from rat cortex after traumatic brain injury (lectin staining with HRP). |
| In addition to the above mentioned role of microglia in neuroinflammation, these cells also play a central role in neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease and Multiple Sclerosis (6). For example in the case of AD, microglial cells become activated in the presence of amyloid beta (Aβ) plaques and are known to have a neuro-protective effect by eliminating Aβ by phagocytosis. Further, the local production of proinflammatory cytokines and chemokines by microglia contribute to the recruitment of circulating lymphocytes to the BBB and increase the permeability of the BBB. Increased lymphocyte infiltration into the brain can then assist microglia in fighting infection and eliminate or prevent formation of the Aβ deposits. However, it seems that in the brain of AD patients, Aβ production exceeds microglia’s capacity for clearance. This results in causing the microglial cells to enter into a non-resolving chronic inflammatory cycle of continuous activation and release of potentially cytotoxic molecules, which contributes to disease progression. Therefore, it remains debatable whether microglial cells have beneficial or detrimental functions in various neuropathological conditions. | |
| Given the importance of microglia in neurodegenerative diseases, a new field of microglial therapeutics has recently emerged, ranging from pharmacologically manipulating existing microglia by switching their M1 and M2 status, to inhibiting microglial activation. Continued research and clinical efforts in the future will help us to improve our understanding of microglial physiology and their roles in neuropathological diseases. Please let us know if you have any suggestions or interesting research experience in this field at tech@biolegend.com. | |
References:
|
|
| Contributed by Mohar Chattopadhyay, Ph.D. | |
 Login / Register
Login / Register 






Follow Us