Apoptosis is a type of programmed cell death used by cells for self elimination. Apoptotic cells do not release their cellular contents and are quickly engulfed by phagocytic cells, and therefore avoid triggering unnecessary inflammatory responses. Plasma membrane blebbing, the destruction of the nucleus and its contents (called karyorrhexis), and budding of the cell into small apoptotic bodies are all morphological characteristics of a cell undergoing apoptosis. This process is regulated by proteases called caspases, as well as signaling proteins from the Bcl-2 family. Since many physiological functions rely on the proper elimination of cells, dysregulation of apoptosis can contribute to diseases like cancer and neurodegeneration. Study the mechanisms of apoptosis and identify apoptotic cells with our toolset of anti-caspase antibodies, recombinant death receptors and ligands, and specialized probes like Apotracker™.

Caspases, which carry out a series of cleavage events that signal for apoptosis, can be categorized into initiator and executioner caspases. When inactive, caspases are called procaspases. Initiator caspases are activated by dimerization, which is driven by several pathways described below. Dimerized initiator caspases will cleave and activate executioner caspases. Executioner caspases then go on to cleave a number of substrates, including cytoskeletal components, membrane proteins, and endonucleases, resulting in DNA destruction and cell death.
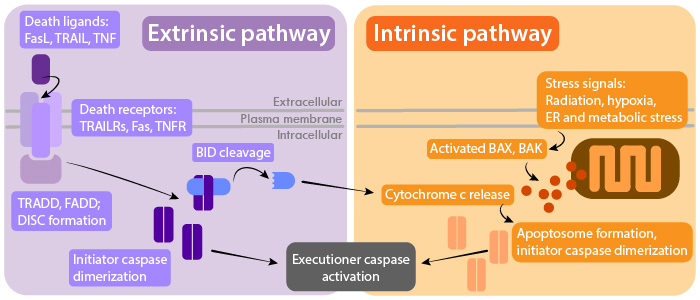
Caspase Activation Pathways
Extrinsic pathway
The extrinsic pathway is regulated by signaling through receptors of the Tumor Necrosis Factor receptor (TNFR) superfamily. These receptors include TNFR, Fas, and TNF-related apoptosis inducing ligand (TRAIL) receptors. Binding of a ligand to its respective receptor activates adaptor proteins that contain death domains, such as FADD and TRADD. Adaptor proteins recruit and facilitate the dimerization of initiator caspases like caspase 8 and 10, which are assembled at the death inducing signaling complex (DISC). This pathway can be negatively regulated by decoy TRAIL receptors (TRAIL R3 and R4) which compete for TRAIL binding.
View products for: TRAIL/TRAILRs | FasL/Fas | TNF | TNFR
Intrinsic pathway
The intrinsic pathway is triggered by detection of cellular stresses, such as DNA damage and hypoxia, or by the absence of signaling from growth factors, hormones, and cytokines. This results in the increased permeability of the mitochondrial membrane, which is regulated by pro- and anti-apoptotic members of the Bcl-2 family (e.g. Bax, Bak, Bad). Cytochrome c is then released from mitochondria to activate adapter protein apoptotic protease-activating factor-1 (APAF1), which forms the apoptosome with dimerized initiator caspase 9. Extrinsic pathway signaling can also cross-activate mitochondrial release of cytochrome c through caspase 8-mediated cleavage of Bid, a pro-apoptotic member of the Bcl-2 family.
View products for: Cytochrome c | Bcl-2
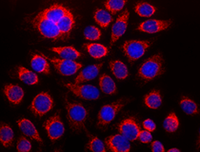
HeLa cells stained with purified anti-Cytochrome c antibody, DyLight 594™-conjugated secondary antibody, and DAPI.
Granzyme pathway
Cytotoxic CD8+ T lymphocytes (CTLs) and natural killer (NK) cells can kill target cells (e.g. cancer cells, infected cells) through FasL/Fas binding, and through secretion of granules containing proteases granzyme A and B. These granules penetrate the target cell membrane through pores formed by perforin. FasL/Fas interaction can trigger extrinsic apoptosis, and is the primary killing mechanism used by NKT cells, a subset of T cells that recognize CD1d. Granzyme B can directly cleave and activate initiator and executioner caspases. Granzyme B can also cleave Bid to induce cytochrome c release and the intrinsic apoptotic pathway. In contrast, granzyme A induces cell death through mechanisms independent of caspases.
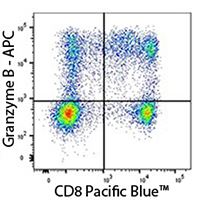
Human PBMC stained with anti-CD8 Pacific Blue™ and anti-Granzyme B APC recombinant antibody.
| Caspase | Description |
|---|---|
|
Inflammatory caspase that cleaves pro-IL-1β to generate the active cytokine |
|
|
The major executioner caspase that can be activated by any of the initiator caspases (8, 9, 10) |
|
|
Executioner caspase that can be cleaved by granzyme B and caspases 3, 9, and 10 |
|
|
Initiator caspase activated by FasL/Fas signaling |
|
|
Initiator caspase activated by the apoptosome and intrinsic pathway |
|
|
Inflammatory caspase activated by LPS or TLR sensing |
References:
- Portt et al. Anti-apoptosis and cell survival: A review. Biochimica et Biophysica Acta (2011). PMID: 20969895.
- McIlwain et al. Caspase Functions in Cell Death and Disease. Cold Spring Harb Perspect Biol (2013). PMID: 23545416
- Pinkoski et al. Granzyme B-mediated Apoptosis Proceeds Predominantly through a Bcl-2-inhibitable Mitochondrial Pathway. J Biol Chem (2001). PMID: 11278459
- Elmore. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol (2007). PMID: 17562483
- Hagar et al. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science (2013). PMID: 24031018
- Galluzzi et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ (2018). PMID: 29362479
- Krijgsman et al. The Role of Natural Killer T Cells in Cancer – A Phenotypical and Functional Approach. Front Immunol (2018). PMID: 29535734
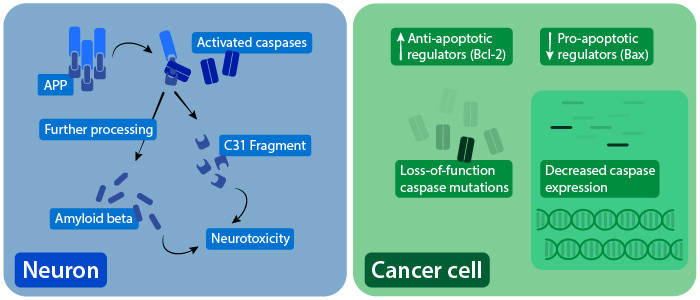
The role of caspases in AD-associated neurotoxicity (left), and dysregulation of apoptosis in cancer cells (right).
Neurodegenerative Disease
Irregular caspase activation has been associated with several neurodegenerative diseases. Caspase activation is elevated in animal models of amyotrophic lateral sclerosis (ALS), and is detected in spinal cord samples from ALS patients. In Huntington’s disease, caspase activation is associated with early disease stages, and caspase expression increases with disease progression. Huntingtin protein (HTT) can also be directly cleaved by caspases, which further contributes to disease features. Evidence also suggests that caspases can cleave amyloid precursor protein (APP), which is processed into neurotoxic components associated with Alzheimer’s disease (AD). In certain AD models, caspase activation precedes neuronal cell death.
Find resources for neurodegeneration research
Cancer
Tumor cells avoid apoptosis by disrupting upstream signaling of caspase pathways. This includes upregulation of anti-apoptotic proteins like Bcl-2, which was originally discovered as an oncogene, and downregulation of pro-apoptotic proteins like Bax. Expression of these apoptotic regulators are controlled by p53, a major tumor suppressor. Tumor cells can also have reduced caspase expression or have loss-of-function caspase mutations. Other methods of apoptosis evasion include decreased expression of Fas and increased expression of decoy Fas receptors that help cancer cells avoid CTL and NK cell-mediated killing.
Find resources for cancer research
Autoimmunity and Inflammation
Decreased apoptosis function can lead to autoimmune disorders. Mutations that lower the function of Fas, FasL, or caspase 10 causes autoimmune lymphoproliferative syndrome (ALPS), which results from the lack of autoreactive T cell death during selection in the thymus. Since caspase 1 activity produces activated IL-1β, caspase 1 has been implicated in a number of inflammatory diseases. Increased IL-1β levels has been associated with type 2 diabetes, gout, and certain types of arthritis.
References:
- Zhang et al. APP Processing in Alzheimer's Disease. Mol Brain (2011). PMID: 21214928
- Bano et al. Neurodegenerative Processes in Huntington's Disease. Cell Death Dis (2011). PMID: 22071633
- McIlwain et al. Caspase Functions in Cell Death and Disease. Cold Spring Harb Perspect Biol (2013). PMID: 23545416
- Friedlander. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med (2003). PMID: 12672865
- Elmore. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol (2007). PMID: 17562483
- Portt et al. Anti-apoptosis and cell survival: A review. Biochimica et Biophysica Acta (2011). PMID: 20969895.
Revolutionize Apoptosis Detection with Apotracker™
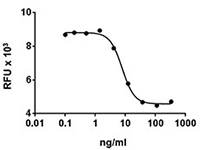
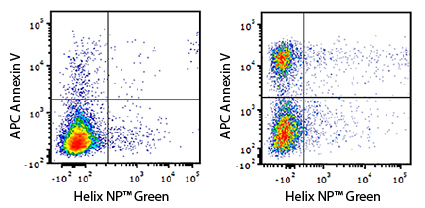
Apotracker™ is a family of fluorogenic probes that detects externalized phosphatidylserine (PS) residues on apoptotic cells in a calcium-independent manner. Traditional apoptosis detection probes, such as Annexin V, require the presence of calcium, which can affect cell viability and marker expression. Apotracker exhibits a linear relationship with Annexin V staining and does not require a specialized buffer. It can also be useful for microscopy assays where it presents less background staining than traditional probes.
Annexin V
Annexin V is a protein that binds to PS, and is commonly used as a probe to identify apoptotic cells. Annexin V binding occurs in a calcium-dependent manner, and its usage requires a binding buffer.
|
|
Annexin A1
Annexin A1 (ANXA1) is another member of the annexin superfamily. ANXA1 has various functions, including inhibition of cytosolic phospholipase A2 (PLA2), regulation of inflammation, and induction of apoptosis. Annexin A1 is expressed on the cell surface during early apoptotic stages.
|
|
 Login / Register
Login / Register 




 Mechanisms of Cell Death Poster
Mechanisms of Cell Death Poster


Follow Us